In this reaction of HBr + CaCO3, calcium carbonate is neutralized by hydrobromic acid. Let us learn more about this reaction.
Calcium carbonate is a calcium salt with the formula CaCO3. It is a fertilizer, food coloring, and food-firming agent. Hydrogen bromide is a colorless gas, normally used as a catalyst, in the production of bromide compounds.
This article studies the various fact of HBr + CaCO3 reactions like its enthalpy of reaction, reaction type, conjugate pairs, product, ionic equation, etc.
What is the product of HBr and CaCO3?
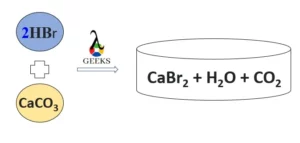
CaCO3 (calcium carbonate) combines with HBr (hydrobromic acid), results in the formation of H2O (water), CO2 (carbon dioxide), and CaBr2 (Calcium bromide).
HBr + CaCO3 → CaBr2 + H2O + CO2
What type of reaction is HBr + CaCO3
It is an Exothermic Reaction and neutralization reaction since, formation of CaBr2 takes place which is a neutral salt.
How to balance HBr + CaCO3
The unbalanced equation of HBr + CaCO3 reaction is given by,
HBr + CaCO3 → CaBr2 + H2O + CO2
- In this case, we have two bromine and two hydrogens on the product side and one each one of them on the reactant side.
- Therefore, we have to change the stoichiometry for HBr into two.
- Balanced Equation: 2HBr + CaCO3 → CaBr2 + H2O + CO2
HBr + CaCO3 conjugate pairs
HBr + CaCO3 reaction has the following conjugate pairs,
- Br- is the conjugate base of HBr because that is what HBr becomes after losing its H+ cation.
- Conjugate acid-base pair for CaCO3 is not possible.
HBr + CaCO3 net ionic equation
- Net ionic equation:
- CaCO3 (s) + 2H+ (aq) →. Ca2+ (aq) + CO2 (g) + H2O (l)
The net ionic equation for CaCO3 + HBr = CaBr2 + CO2 + H2O is written in three steps:
- Beginning with balancing the molecular equation.
- 2HBr + CaCO3 → CaBr2 + H2O + CO2
- Secondly, write the states and separate the soluble ionic compounds into their ions (these are the strong electrolytes denoted by an (aq)). Complete ionic equation:
- CaCO3 (s) + 2H+ (aq) + 2Br– (aq) → Ca2+ (aq) + 2Br– (aq) + CO2 (g) + H2O (l)
- Finally, any spectator ions are removed. These ions can be found on both sides of the ionic equation.
HBr and CaCO3 intermolecular forces
The forces between HBr and CaCO3 are as follows:
• Forces acting on HBr include permanent dipole-dipole, dispersion forces and hydrogen bonding.
• In the molecules of CaCO3, there is an electrostatic attraction.
HBr + CaCO3 reaction enthalpy
HBr + CaCO3 exhibits a reaction enthalpy of -25.67 kJ.
• The following is a table of the reaction enthalpies of formation of each component on either side of the reaction:
| Compound | Moles | Enthalpies |
|---|---|---|
| CaCO3(aq) | 1 mol | -1219.97kJ/mol |
| HBr(g) | 2 mol | –36.23 kJ/mol |
| CaBr2(s) | 1 mol | -682.8 kJ/mol |
| CO2(g) | 1 mol | -393.5 kJ/mol |
| H2O(g) | 1 mol | -241.8 kJ/mol |
• Change in reaction enthalpy = Sum of enthalpies in product side – sum of enthalpies in reactant side.
• Enthalpy change = (-1219.97-36.23) – (-682.8-393.5-241.8) = -25.67 kJ/mol.
Is HBr + CaCO3 a buffer solution
HBr + CaCO3 is not a buffer solution because HBr is a strong acid since its pKa value is -9. Calcium carbonate is a salt that is both a strong base due to the presence of the calcium ion and weak acid because of the presence of the carbonate ion and is typically derived from carbonic acid.
Is HBr + CaCO3 a complete reaction
HBr + CaCO3 is a complete reaction because all the products formed are stable.
Is HBr + CaCO3 an exothermic or endothermic reaction
HBr + CaCO3 is exothermic since, ΣΔH°f(reactants) > ΣΔH°f(products).
Is HBr+ CaCO3 a redox reaction
HBr + CaCO3 is not a redox reaction, because oxidation and reduction reactions do not occur concurrently in the process,
Is HBr + CaCO3 a precipitation reaction
HBr + CaCO3 is not a precipitation reaction, CaBr2 is not a salt.
Is HBr + CaCO3 reversible or irreversible reaction
HBr + CaCO3 is irreversible reaction because the products do not revert to the original reactant at constant operating conditions.
Is HBr + CaCO3 displacement reaction
HBr + CaCO3 is not a displacement reaction since formation of new compound takes place without exchange of ions.
Conclusion
Reaction of hydrogen bromide with calcium carbonate involves formation of neutral salt, CaBr2, gas and liquid water. CaBr2 is used as dense aqueous solutions in drilling fluids, used as food preservatives and in photography. CO2 is a crucial industrial material used as inert gas in pressurizing gas in oil refinery.

Hello folks, myself Gauri Ladda from the Institute Of Chemical Technology Mumbai, Marthwada Campus Jalna. With Lambdageeks I am looking forward to sharing my chemical engineering skills and knowledge with the readers.