Sulphuric acid(H2SO4) is an inorganic an viscous liquid compound and Zinc chloride (ZnCl2) is an inorganic white mineral. Let us discuss the reactions of H2SO4+ ZnCl2.
H2SO4 is a colorless acid that absorbs moisture from the air and is also known as oil of vitriol, kept in glass bottle because of the high reactivity. ZnCl2 is a white crystalline powder and hygroscopic in nature with high solubility in acids like HCl and alkaline solutions with the density of 2.907 g/cm3.
In this article, we are going to take a look at the following sections enthalpies, conjugate pairs, products, and net ionic reaction with the type of reaction for H2SO4+ ZnCl2.
What is the product of H2SO4 and ZnCl2
Zinc sulphate (ZnSO4) and hydrochloric acid (HCl) are the products of H2SO4+ ZnCl2.
H2SO4+ ZnCl2= ZnSO4 + HCl
What type of reaction is H2SO4+ ZnCl2
H2SO4+ ZnCl2 is a double replacement reaction as the dissociative ions present in liquid medium displaces the ions of both the reactants to form the products.
How to balance H2SO4+ ZnCl2
The equation H2SO4+ ZnCl2 is balanced by using the following steps–
H2SO4+ ZnCl2 = ZnSO4 + 2HCl
- Name each reactant and product with a specific alphabet like A, B, C, and D.
- A H2SO4+ B ZnCl2= C ZnSO4 + D HCl
- Modify the atoms with suitable numbers.
- H –> A,D S –>A, C Cl –>B,D Zn–>B,C
- Multiply the coefficients with suitable numbers.
- A=1, B = 1 ,C = 1 ,D = 2
- Now, reduce the value of the lowest integer.
- So, the final equation is
- H2SO4+ ZnCl2 = ZnSO4 + HCl
H2SO4+ ZnCl2 titration
H2SO4 cannot be titrated with ZnCl2 because the product of the reaction is ZnSO4 and HCl are acids that increase the acidity of the solution, due to which it is not possible to calculate the unknown concentration H2SO4+ ZnCl2 and endpoint of the solution.
H2SO4+ ZnCl2 net ionic equation
The net ionic reaction of H2SO4+ ZnCl2 is –
H+ + SO4- + Zn+ + Cl– = Zn+ + SO4- + H+ + Cl–
Following steps should be followed to write the net ionic equation.
- Write the reaction with states.
- H2SO4 (l) + ZnCl2 (s)= ZnSO4 (l) + HCl (l)
- Splits the atoms into ions.
- Thus, net ionic equation is –
- H+ + SO4- + Zn+ + Cl– = Zn+ + SO4- + H+ + Cl–
H2SO4+ ZnCl2 conjugate pairs
H2SO4+ ZnCl2 has the following conjugate pairs–
- H2SO4 conjugate acid has HSO4- conjugate base after the deprotonation.
- ZnCl2 conjugate base has HCl as a conjugate acid after protonation.
H2SO4 and ZnCl2intermolecular forces
The intermolecular forces present between H2SO4+ ZnCl2 are –
- Dipole-dipole interaction force and strong electrostatic force with intermolecular hydrogen bonds are the intermolecular forces present between the molecules of H2SO4 .
- Intermolecular hydrogen bonds with covalent bonds are the intermolecular forces of attraction present between the atoms of ZnCl2.
H2SO4+ ZnCl2 reaction enthalpy
The reaction enthalpy of H2SO4+ ZnCl2 is -814 KJ/mole, where-
- Enthalpy of formation of H2SO4 = -814 KJ/mole
- Enthalpy of formation of ZnCl2 = 0 KJ/mole
- Reaction enthalpy is calculated as = Enthalpy of formation of H2SO4 – Enthalpy of formation of ZnCl2
- = -814 KJ/mole – 0 KJ/mole
- = -814 kJ/mole
Is H2SO4+ ZnCl2 a buffer solution
H2SO4+ ZnCl2 is not a buffer solution because the products of the reaction are ZnSO4 and HCl which can make the buffer solution acidic due to which the pH of the solution does not specify the pH of the buffer solution.
Is H2SO4+ ZnCl2 a complete reaction
H2SO4+ ZnCl2 is a complete reaction as the products are zinc sulfate (ZnSO4) and hydrochloric acid (HCl) which are the complete complex of a chemical species and cannot react further to make any other compound.
Is H2SO4+ ZnCl2 an exothermic or endothermic reaction
H2SO4+ ZnCl2 is an exothermic reaction it is because when ZnCl2 is added to H2SO4 the process of bond dissociation of ZnCl2 and H2SO4 takes place due to the high reactivity of H2SO4 and this leads to production of large amount of heat which warms up the solution.
Is H2SO4+ ZnCl2 a redox reaction
H2SO4+ ZnCl2 is not a redox reaction because the oxidation states of reactants and products are same throughout the reaction as there is no exchange of electrons and protons.
Is H2SO4+ ZnCl2 a precipitation reaction
H2SO4+ ZnCl2 is not a precipitation reaction as the solubility of ZnSO4 is very high because of the covalent bond present between them, due to which it dissolves immediately as comes in contact with H2SO4 and does not form any precipitated salt.
Is H2SO4+ ZnCl2 reversible or irreversible reaction
H2SO4+ ZnCl2 is an irreversible reaction as the product of the reaction ZnSO4inc sulfate is a complete complex of an inorganic chemical compound and cannot be reversed in the form of a reactant again.
Is H2SO4+ ZnCl2 displacement reaction
H2SO4+ ZnCl2 is a double-displacement reaction as the dissociation of ions takes place in the reaction. Here, the respective ion H+ displaces the Zn+ ion to form HCl (hydrochloric acid), and the SO4- ion displaces the Cl– ion to form ZnSO4 (zinc sulfate).
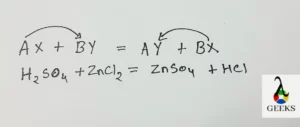
Conclusion
H2SO4 is used as a reagent for salt analysis and for refining petrol as well as for making fertilizers. Whereas, ZnCl2 can be used in various chemical syntheses because of the high solubility and high melting points with processing in textile industries and in fixing of metallurgical fluxes.

Hi….I am Soumya Chourasia, completed my Master’s in Chemistry. I am working as a Subject Matter Expert in Chemistry. Before this, I used to teach chemistry for competitive examinations and school as well.
Let’s connect through LinkedIn here,