P4O10 is a strong desiccating or dehydrating agent even stronger than H2SO4. Let us see how it reacts with H2SO4 through this article.
Sulphuric acid (H2SO4) and tetraphosphorus decaoxide react to form acid and release gas. Tetraphosphorus decaoxide (P4O10) shows similar chemical properties like P2O5. Both are strong dehydrating agents of organic chemistry. But only P4O10 is strong enough to dehydrate H2SO4.
We will learn some useful facts about the H2SO4 + P4O10 reaction, like type of reaction, products formed, balanced chemical equation and reaction enthalpy in this article.
What is the product of H2SO4 and P4O10
Phosphoric acid (H3PO4) and sulphur trioxide (SO3) are the products of the H2SO4 + P4O10 reaction. The chemical equation for the reaction is,
H2SO4 + P4O10 = H3PO4 + SO3
What type of reaction is H2SO4 + P4O10
H2SO4 + P4O10 reaction is a double displacement reaction.
How to balance H2SO4 + P4O10
The balanced chemical equation for the H2SO4 + P4O10 reaction is,
6H2SO4 + P4O10 = 4H3PO4 + 6SO3
The following steps are used in derivation of the balanced chemical equation:
- The unbalanced equation for H2SO4 + P4O10 reaction is,
- H2SO4 + P4O10 = H3PO4 + SO3
- To check if the moles of atoms present on the product side are equal to the reactant side or not. Here the number of H, P, O and S atoms are not equal.
- Therefore, we multiply H2SO4, H3PO4 and SO3 with a coefficient of 6, 4 and 6 respectively.
- Thus, the balanced chemical equation obtained is,
- 6H2SO4 + P4O10 = 4H3PO4 + 6SO3
H2SO4 + P4O10 titration
Titration of H2SO4 and P4O10 is not possible as sulphuric acid is a strong acid and tetraphosphorus decaoxide is an acidic oxide that cannot be categorised in any titration procedure.
H2SO4 + P4O10 net ionic equation
The net ionic equation for H2SO4 + P4O10 reaction is,
2H+ (aq.) + SO42- (aq.) + P4O10 (aq.) = 2H+ (aq.) + PO32- (aq.) + SO3 (g)
The steps mentioned below are used in the derivation of the net ionic equation:
- Note the general balanced chemical equation for H2SO4 and P4O10 reaction.
- 6H2SO4 + P4O10 = 4H3PO4 + 6SO3
- Specify each molecule with its chemical state (s, l, g or aq).
- 6H2SO4 (aq.) + P4O10 (aq.) = 4H3PO4 (aq.) + 6SO3 (g)
- Split the compounds which are strong enough to produce corresponding ions in their aqueous form to obtain the complete ionic equation.
- 2H+ (aq.) + SO42- (aq.) + P4O10 (aq.) = 2H+ (aq.) + PO32- (aq.) + SO3 (g)
- Eliminate the ions that are available on both sides.
- SO42- (aq.) + P4O10 (aq.) = PO32- (aq.) + SO3 (g)
H2SO4 + P4O10 conjugate pairs
H2SO4 and P4O10 do not have any conjugate pairs.
- The conjugate pair of H2SO4 is its conjugate base HSO42-.
H2SO4 and P4O10 intermolecular forces
- The intermolecular force in H2SO4 molecules is hydrogen bonding.
- Strong van der Waals forces are present in P4O10 molecules.
- H3PO4 also contains hydrogen bonding between their molecules.
- SO3 is a gaseous molecule and has London dispersion forces in-between molecules.
H2SO4 + P4O10 reaction enthalpy
H2SO4 + P4O10 reaction enthalpy is 4955.7 kJ/mol. The estimate values of standard enthalpy of formation for molecules in the reaction is,
| Molecules | Reaction enthalpy (in KJ/mol) |
|---|---|
| H2SO4 | -909.27 |
| P4O10 | -2984 |
| H3PO4 | -277.4 |
| SO3 | -395.72 |
Reaction enthalpy (ΔHf) = Standard enthalpy of formation (product – reactant)
Thus, ΔHf = [6*(-395.72) + 4*(-277.4)] – [-2948 + 6*(-909.27)
ΔHf = 4955.7 kJ/mol.
Is H2SO4 + P4O10 a buffer solution
H2SO4 + P4O10 is not a buffer solution because a weak acid is necessary for forming a buffer solution but H2SO4 is a strong acid.
Is H2SO4 + P4O10 a complete reaction
H2SO4 + P4O10 is a complete reaction and cannot be proceeded further.
Is H2SO4 + P4O10 an exothermic or endothermic reaction
H2SO4 + P4O10 reaction is an endothermic reaction as the reaction enthalpy has a high positive value here.
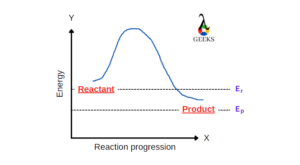
Is H2SO4 + P4O10 a redox reaction
H2SO4 + P4O10 reaction is not a redox reaction as the oxidation states of all the atoms are unchanged during the whole process.
Is H2SO4 + P4O10 a precipitation reaction
The H2SO4 + P4O10 reaction is not a precipitation reaction as no solid phase compound is formed at the end of this reaction.
Is H2SO4 + P4O10 reversible or irreversible reaction
H2SO4 + P4O10 reaction is an irreversible reaction because we cannot add the SO3 gas evolved during the process back into the reaction mixture.
Is H2SO4 + P4O10 displacement reaction
H2SO4 + P4O10 reaction is a double displacement reaction in which Hydrogen and Phosphorus atom displace each other to form new compounds.
Conclusion:
This article concludes that both H2SO4 and P4O10 are very important compounds of chemistry. H2SO4 is a strong dehydrator and P4O10 is a stronger version of H2SO4. P4O10 reacts vigorously with water to produce phosphoric acid.

Hi, I am Sahil Singh. I completed my graduation in Bachelor of Science. I always have keen interest in Physics & Chemistry. I worked on my own blog for 1 year in the technology and gaming niche. I try my best to provide valuable knowledge through my articles.
You can reach out to me on LinkedIn: