Sulphuric acid reacts with chlorous acid, sodium salt forms four different compounds. Let us study the reaction of H2SO4 + NaClO2 in detail.
Sulfuric acid is a viscous, colorless, and miscible in water, odorless liquid. It is an important commodity and industrial compound, produced from various different methods. Sodium chlorite is usually found in industrial setting as a detergent and as bleach. It is produced from two ways reduction and electrolysis.
The following article studies the various fact of H2SO4 + NaClO2 reactions like the reaction type, product, conjugate pairs, enthalpy of reaction, ionic equation etc.
What is the product of H2SO4 and NaClO2?
Sulfuric acid (H2SO4) reacts with sodium chlorite (NaClO2) to form chlorine dioxide (ClO2), sodium sulfate (Na2SO4), water (H2O) and sodium chloride (NaCl). Any one of the following reaction may occur,
NaClO2 + H2SO4 → ClO2 + Na2SO4 + H2O + NaCl
OR,
2NaClO2 + H2SO4 → Na2SO4 + 2HClO2
What type of reaction is H2SO4 + NaClO2?
H2SO4 + NaClO2 is a gas evolution reaction, redox reaction and precipitate reaction.
How to balance H2SO4 + NaClO2?
To balance the given chemical reaction following steps are followed:
- The unbalanced chemical reaction is written as:
- H2SO4 + NaClO2 → ClO2 + Na2SO4 + H2O + NaCl
- The respective number of moles of each element are tabulated as shown below :
| Elements | Reactant Side | Product Side |
|---|---|---|
| Na | 1 | 3 |
| Cl | 1 | 2 |
| S | 1 | 1 |
| O | 6 | 7 |
| H | 2 | 2 |
- The given reaction is balanced when number of moles of Na, Cl, S, O and H are balanced on either side of the reaction.
- Here moles of each elements are unbalanced on either side.
- To balance the reaction, following multiplication is carried out:
- Multiply 2 with H2SO4 , reactant side
- Multiply 5 with NaClO2, reactant side
- Multiply 4 with ClO2, reactant side
- Multiply 2 each with Na2SO4 and H2O on product side
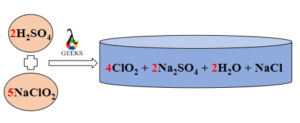
- The Balanced chemical reaction is
- 2H2SO4 +5NaClO2 → 4ClO2 + 2Na2SO4 + 2H2O + NaCl
H2SO4 + NaClO2 titration
H2SO4 + NaClO2 titration is not possible since the reaction leads to the formation of gas, ClO2 .
H2SO4 + NaClO2 net ionic equation
The net ionic equation is – 5ClO2–(aq) + 4H+(aq) = 4ClO2(g) + 2H2O(l) + Cl–(aq)
- First, the phase or state of each compound is determined as shown below:
- 5NaClO2(aq) + 2H2SO4(aq) = 4ClO2(g) + 2Na2SO4(aq) + 2H2O(l) + NaCl(aq)
- Second, complete ionic equation is written by separating the ionic compounds into their respective ions,
- 5Na+(aq) + 5ClO2–(aq) + 4H+(aq) + 2SO42-(aq) = 4ClO2(g) + 4Na+(aq) + 2SO42-(aq) + 2H2O(l) + Na+(aq) + Cl–(aq)
- The common ions present in the both reactant and product sides are removed.
5Na+(aq) + 5ClO2–(aq) + 4H+(aq) +2SO42-(aq) = 4ClO2(g) +4Na+(aq) +2SO42-(aq) + 2H2O(l) +Na+(aq) + Cl–(aq)- The net ionic equation involves compounds which are taking place in the reaction.
- The net ionic equation is –
- 5ClO2–(aq) + 4H+(aq) = 4ClO2(g) + 2H2O(l) + Cl–(aq)
H2SO4 + NaClO2 conjugate pairs
The conjugate acid-base pairs for H2SO4 + NaClO2 are,
- Conjugate acid of H2SO4 = H3O+
- Conjugate base of H2SO4 = HSO4–
- Conjugate acid of NaClO2 = HClO+
- Conjugate base of NaClO2 = ClO2–
H2SO4 + NaClO2 intermolecular forces
The intermolecular forces on H2SO4 and NaClO2 are-
- Van der Waals dispersion forces, Dipole-dipole interaction, and Hydrogen bond acts on H2SO4.
- Ion- dipole forces act on NaClO2
H2SO4 + NaClO2 reaction enthalpy
H2SO4 + NaClO2 change in reaction enthalpy is, -1141.16 kJ/mol.
- The reaction enthalpy of formation of each compound on the either side of the reaction is tabulated down as:
| Compounds | Enthalpy (kJ/mol) |
|---|---|
| H2SO4 | -909.27 |
| NaClO2 | -307 |
| Na2SO4 | -1387.1 |
| ClO2 | 102.5 |
| NaCl | -787 |
| H2O | -285.83 |
- Change in reaction enthalpy = Sum of enthalpies in product side – sum of enthalpies in reactant side.
- Change in Enthalpy = (-1387.1+102.5-787-285.83) – (-909.27-307) = -1141.16 kJ/mol
Is H2SO4 + NaClO2a buffer solution?
H2SO4 + NaClO2 is not a buffer solution due to the presence of strong H2SO4 acid in the reaction.
Is H2SO4 + NaClO2 a complete reaction?
H2SO4 + NaClO2 is defined as a complete reaction since moles of reactant are completely consumed to yield products at equilibrium.
Is H2SO4 + NaClO2 an exothermic or endothermic reaction?
H2SO4 + NaClO2 is an exothermic reaction because the change in enthalpy is -1141.16 kJ/mol, which is negative thereby increasing the temperature.
Is H2SO4 + NaClO2 a redox reaction?
H2SO4 + NaClO2 is a redox reaction.
- Reduction, ClIII + 4e– → Cl-I
- Oxidation, 4ClIII – 4e– → 4ClIV
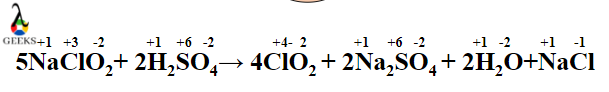
Is H2SO4 + NaClO2 a precipitation reaction?
H2SO4 + NaClO2 is a precipitation reaction, due to the formation of salt NaCl during the reaction.
Is H2SO4 + NaClO2 reversible or irreversible reaction?
H2SO4 + NaClO2 is not a reversible reaction, unless and until there is drastic change in experiment pressure or temperature.
Is H2SO4 + NaClO2 displacement reaction?
H2SO4 + NaClO2 reaction is a double displacement (metathesis) reaction, as Na is displaced twice to form the product.
Conclusion
Reaction of sulfuric acid with sodium chlorite involves formation of gas (ClO2) and formation of salt (NaCl). Sodium sulfate is mainly used as filler in production of detergents, in Kraft process of paper pulping. Sodium chloride is used as feedstocks for different chemical synthesis and is an important industrial chemical.
Hi….I am Pratham Manish Shah, Pursuing an Integrated MTech degree in Chemical Engineering from the Institute of Chemical Technology Mumbai Marathwada Jalna. With Lamdageeks, I am Interested in learning ongoing education opportunities to maintain knowledge of emerging technologies and methods.
Let’s connect via LinkedIn: www.linkedin.com/in/pratham-shah-07