H2SO4 is a strong dehydrating agent and an acid. Sodium (Na) is an alkali metal. Let us look at some reactions between H2SO4 and Na in this article.
Na is a reactive metal that upon reacting with H2SO4 produces salt and gas. Sulphuric acid (H2SO4) is a compound composed of oxygen, sulphur and hydrogen. Concentrated H2SO4 can act as a strong water-removal agent in reactions. Sodium (Na) metal is highly reactive and therefore is not available in a free state.
This article, will discuss important facts about the H2SO4+Na reaction like precipitation, type of reaction and balanced chemical equation.
What is the product of H2SO4 and Na
Sodium sulphate (Na2SO4) and hydrogen gas (H2) are the products of H2SO4 and Na reaction. The chemical equation for H2SO4 + Na reaction is as follows:
H2SO4 + Na = Na2SO4 + H2
What type of reaction is H2SO4 and Na
H2SO4 + Na reaction is a single displacement reaction.
How to balance H2SO4 and Na
H2SO4 + Na balanced chemical equation is,
H2SO4 + 2Na = Na2SO4 + H2
- The general chemical equation for the above reaction is
- H2SO4 + Na = Na2SO4 + H2
- To check whether the number of atoms on the reactant side is equal to number of atoms available on the product side.
- Only Na atoms are not equal here, therefore we multiply a coefficient of 2 with Na on reactant side.
- Thus, the balanced chemical equation is
- H2SO4 + 2Na = Na2SO4 + H2
H2SO4 and Na titration
Titration of H2SO4 with Na is not feasible because H2SO4 is an acid but Na is an elementary metal and not a base.
H2SO4 and Na net ionic equation
H2SO4 + Na reaction net ionic equation is,
2Na (s) + 2H+ (aq.) + SO42- (aq.) = 2Na+ (aq.) + SO42- (aq.) + H2 (g)
- Write the general balanced chemical equation for the reaction is
- 2Na + H2SO4 = Na2SO4 + H2
- Denote chemical states of each compound involved during the reaction.
- 2Na (s) + H2SO4 (aq.) = Na2SO4 (aq.) + H2 (g)
- Split the strong electrolytes into their respective ions.
- 2Na (s) + 2H+ (aq.) + SO42- (aq.) = 2Na+ (aq.) + SO42- (aq.) + H2 (g)
- Cancel out the spectator ions from the above equation to get the net ionic equation for H2SO4 + Na
- 2Na (s) + 2H+ (aq.) = 2Na+ (aq.) + H2 (g)
H2SO4 and Na conjugate pairs
H2SO4 + Na reaction has the following conjugate pairs,
- Conjugate pair of Na is not possible, as it is an elementary metal.
- Conjugate pair of H2SO4 is its conjugate base HSO4–.
H2SO4 and Na intermolecular forces
H2SO4 + Na reaction has the following intermolecular forces,
- The intermolecular force between H2SO4 is hydrogen bonding. That is why sulphuric acid is miscible in water.
- Ionic interactions are present in Na2SO4 because it is an ionic compound.
- H2 molecules contain dipole-dipole interactions.
H2SO4 + Na reaction enthalpy
H2SO4 and Na reaction enthalpy is -480.2 kJ/mol. Standard formation enthalpy of reactants and products involved in reaction are:
| Molecules | Reaction enthalpy (in kJ/mol) |
|---|---|
| Na | 0 |
| H2SO4 | -909.27 |
| Na2SO4 | -1389.51 |
| H2 | 0 |
ΔfH: Standard enthalpy of formation of products – Standard enthalpy of formation of reactants
ΔfH: [ -1389.51 -0] – [-909.27]
ΔfH: -480.2 kJ/mol
Is H2SO4 + Na a buffer solution
H2SO4 + Na reaction is not a buffer solution because H2SO4 is a strong acid, whereas for a buffer solution we need a weak acid or weak base.
Is H2SO4 + Na a complete reaction
H2SO4 + Na reaction is a complete reaction where H2SO4 and Na react to form Na2SO4 and H2
Is H2SO4 + Na an exothermic or endothermic reaction
H2SO4 and Na reaction is an exothermic reaction because the reaction enthalpy has a negative value and heat will be released during the reaction.
Is H2SO4 + Na a redox reaction
H2SO4 + Na reaction is a redox reaction where hydrogen is getting reduced and sodium is getting oxidised.
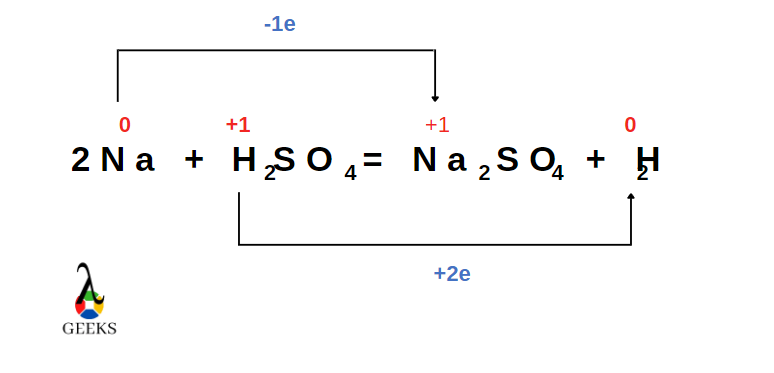
Is H2SO4 + Na a precipitation reaction
H2SO4 + Na reaction is not a precipitation reaction because soluble and gaseous products are formed during the reaction.
Is H2SO4 + Na reversible or irreversible reaction
H2SO4 + Na reaction is an irreversible reaction because the reaction path is one way and the H2 gas evolved cannot be reverted back into the reaction.
Is H2SO4 + Na displacement reaction
H2SO4 + Na reaction is a single displacement or substitution reaction where H2 is getting displaced from H2SO4 by Na atom.
Conclusion
In the end, we can conclude that H2SO4 reacts with calcium to produce Na2SO4 and H2. Na metal is highly reactive and should be kept away from moisture. H2 is a highly flammable gas and that is why handled with care.

Hi, I am Sahil Singh. I completed my graduation in Bachelor of Science. I always have keen interest in Physics & Chemistry. I worked on my own blog for 1 year in the technology and gaming niche. I try my best to provide valuable knowledge through my articles.
You can reach out to me on LinkedIn: