Sulphuric acid or H2SO4 appears as a colorless oily liquid, and MnS is a sulfide salt of manganese. Let us explore the several facets of the reaction between these two compounds.
Sulphuric acid is also known as the king of acids because of its huge application in manufacturing different chemicals. Manganese sulfide appears as a brown, green or red-colored powder. The crystals of MnS have a rock salt type of structure.
As we continue, all the essential features of the reaction between H2SO4 and MnS will come to light.
What is the product of H2SO4 and MnS
Manganese sulfate (MnSO4) and hydrogen sulfide (H2S) gas are the products of the reaction H2SO4 + MnS.
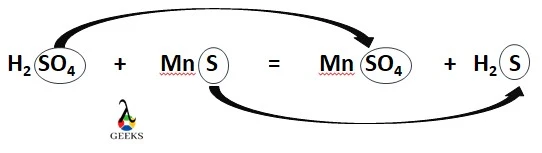
H2SO4 (aq) + MnS (s) = MnSO4 (aq) + H2S (g)
What type of reaction is H2SO4 and MnS
H2SO4 + MnS belongs to the category of displacement reaction.
How to balance H2SO4 and MnS
The reaction H2SO4 + MnS is balanced using the following steps-
H2SO4 + MnS = MnSO4 + H2S
- The reaction is already balanced as the number of atoms of H, S, O, and Mn are intact after the reaction.
| Element | Before reaction | After reaction |
|---|---|---|
| H | 2 | 2 |
| S | 2 | 2 |
| O | 4 | 4 |
| Mn | 1 | 1 |
H2SO4 and MnS titration
Titration between H2SO4 and MnS is not possible as this is a reaction between an acid with a sulfide salt producing a sulfate salt and an acid.
H2SO4 and MnS net ionic equation
The net ionic equation for H2SO4 + MnS is
2H+ (aq) + MnS (s) = Mn2+ (aq) + H2S(g)
These are the steps to be followed to derive the net ionic equation.
- Write the respective ions only for soluble ionic compounds.
- H2SO4 and MnSO4 being ionic, dissociate into cations and anions.
- The complete ionic equation is
- 2H+ (aq) + SO42– (aq) + MnS (s) = Mn2+ (aq) + SO42- (aq) + H2S (g)
- SO42- ion being the spectator ion here, will be canceled from both sides.
- Thus the net ionic equation is
- 2H+ (aq) + MnS (s) = Mn2+ (aq) + H2S(g)
H2SO4 and MnS conjugate pairs
- Sulfate ion (SO42-) is the conjugate base of the acid H2SO4.
- MnS does not have a conjugate pair as it is a metal sulfide.
H2SO4 and MnS intermolecular forces
- H-bonding and dipole-dipole these two intermolecular forces are present in H2SO4 molecule where the first one is more significant.
- Electrostatic force of attraction will be there in MnS as it is ionic in nature.
H2SO4 and MnS reaction enthalpy
For H2SO4 + MnS, the reaction enthalpy value is -27.2 kJ/mole.
| Compounds | Enthalpy of formation (ΔHf°) in kJ/mole |
|---|---|
| H2SO4 (aq) | -909.3 |
| MnS (s) | -214.2 |
| MnSO4 (aq) | -1130.1 |
| H2S(g) | -20.6 |
- Reaction Enthalpy = ΣΔHf°(products) – ΣΔHf° (reactants)
- = [(-1130.2) + (-20.6)] – [(-909.3) + (-214.2)] KJ/mol
- = -27.2 KJ/mol
Is H2SO4 and MnS a buffer solution
H2SO4 + MnS cannot be a buffer solution as H2SO4 is a strong acid.
Is H2SO4 and MnS a complete reaction
H2SO4 + MnS is a complete reaction, as there is no further reaction once MnSO4 and H2S are formed.
Is H2SO4 and MnS an exothermic or endothermic reaction
H2SO4 + MnS is exothermic in nature as -27.2 kJ/mole heat is released.
Is H2SO4 and MnS a redox reaction
H2SO4 + MnS is not a redox reaction as oxidation states are the same for the atoms H, S, O, and Mn before and after the reaction.
Is H2SO4 and MnS a precipitation reaction
H2SO4 + MnS is not a precipitation reaction as the product MnSO4 is a water-soluble compound, and the other product is a gas.
Is H2SO4 and MnS reversible or irreversible reaction
H2SO4 + MnS is an irreversible reaction as it is a unidirectional reaction.
Is H2SO4 and MnS displacement reaction
H2SO4 + MnS is a displacement reaction as SO42- and S2- ions are interchanged between two reactant molecules, H2SO4 and MnS.
Conclusion
In conclusion, the product MnSO4 can form a variety of hydrates, all of which can dissolve in water producing a faint pink-colored solution. The other product, H2S, is a colorless, pungent-odoured gas.

Hello, I am Tuluma Das, Completed my Ph.D. in Organic Chemistry from the Indian Association for the Cultivation of Science. I have a total of 9 years of research experience including a Ph.D. and Postdoc and 3 years of teaching experience. I have published 7 papers so far in international journals. Let’s connect through Linkedin :