H2O’s Lewis structure features an oxygen atom with two lone pairs and two single bonds connecting to hydrogen atoms, forming a bent molecular geometry. The oxygen atom has six valence electrons, sharing two with hydrogens (each contributing one electron) to complete its octet, resulting in a bond angle of 104.5°, slightly less than the tetrahedral angle due to lone pair repulsion, following the VSEPR theory for its AX2E2 configuration.
How to Draw Lewis Structure of H2O
Water (H2O) is a molecule composed of two hydrogen atoms bonded to a central oxygen atom. The Lewis structure helps us understand the bonding and electron distribution in water, which is essential for understanding its chemical properties.

Steps to Draw the Lewis Structure of H2O
Follow these steps to draw the Lewis structure of H2O:
Step 1: Count the Total Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom. To determine the total number of valence electrons in H2O, add up the valence electrons of each atom.
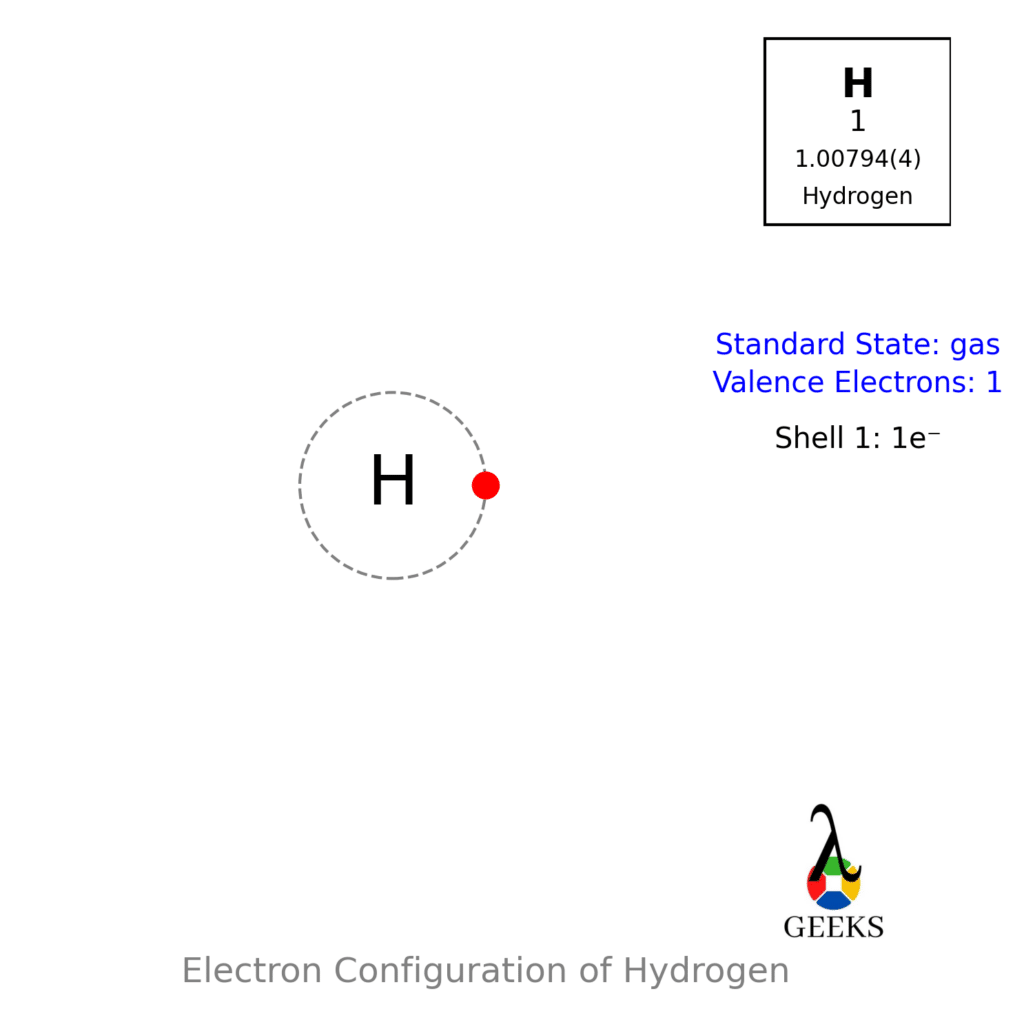
- Hydrogen (H) has 1 valence electron.
- Oxygen (O) has 6 valence electrons.
Since there are two hydrogen atoms and one oxygen atom in H2O, the total number of valence electrons is 2(1) + 6 = 8.
Step 2: Identify the Central Atom
In H2O, the oxygen atom is more electronegative than hydrogen, so it will be the central atom. The hydrogens will be the outer atoms.
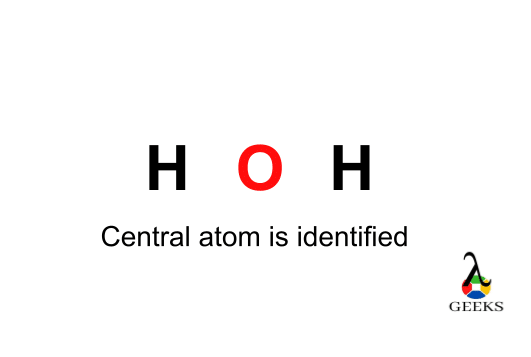
Step 3: Connect the Atoms with Electron Pairs
Draw a single bond between the oxygen atom and each hydrogen atom to represent the sharing of electrons. Each bond consists of a pair of electrons.

Step 4: Distribute the Remaining Electrons
In H2O, we have used 2 electrons for the bonds, leaving us with 8 – 2 = 6 valence electrons. These remaining electrons will be placed on the central oxygen atom.


Step 5: Check the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with 8 electrons in their outermost shell.
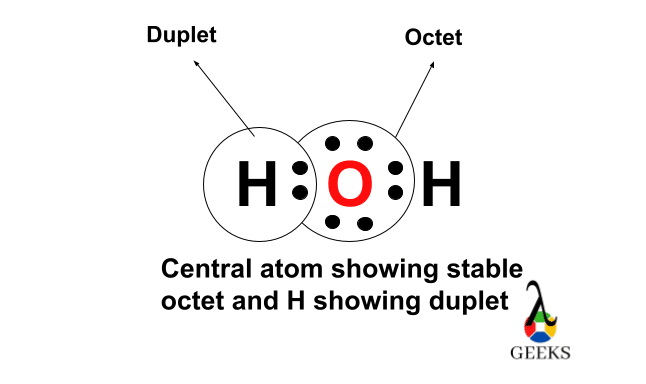
In the Lewis structure of H2O, the oxygen atom has 6 valence electrons from step 1, plus the 6 non-bonding electrons from step 4, giving it a total of 12 electrons.
Since oxygen can accommodate a maximum of 8 valence electrons, we need to move a lone pair of electrons from the oxygen atom to form a double bond between the oxygen and hydrogen atoms.
Step 6: Finalize the Lewis Structure
After moving one of the lone pairs, the oxygen atom will have a total of 8 valence electrons, and each hydrogen atom will have 2 valence electrons.
The final Lewis structure of H2O is as follows:
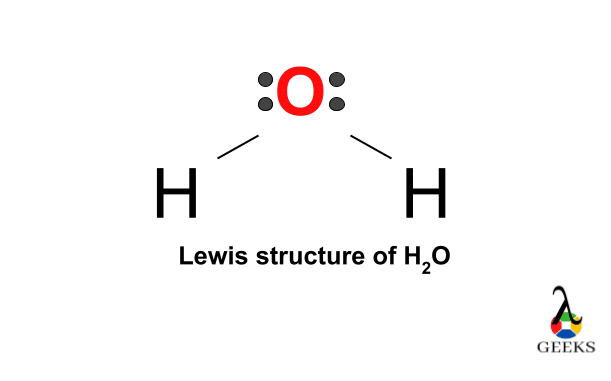
Drawing the Lewis structure of H2O helps us visualize the arrangement of atoms and valence electrons in the molecule. It allows us to understand the bonding and electron distribution, which are crucial for understanding the chemical behavior of water.
Remember, the Lewis structure is a simplified representation, and the actual electron distribution may differ. However, the Lewis structure provides a useful framework for understanding chemical bonding.
Also Read:
- Nf4 lewis structure
- Li2o lewis structure
- Nh2cooh lewis structure
- Pi5 lewis structure
- Brf5 lewis structure
- Hno lewis structure
- Bei2 lewis structure
- Hclo lewis structure
- Sbh3 lewis structure
- Sp3 lewis structure

Hello everyone, I am Dr. Shruti M Ramteke, I did my Ph.D. in chemistry. I am passionate about writing and like to share my knowledge with others . Feel free to contact me on linkedin