Summary
Glucose solubility is a critical parameter that determines the maximum amount of glucose that can be dissolved in a given volume of solvent at a specific temperature. The solubility of glucose in water is remarkably high, making it an ideal candidate for various applications, including the food industry, pharmaceuticals, and biofuel production. However, the solubility of glucose in other solvents, such as ethanol-water mixtures and ionic liquid (IL) + antisolvent mixtures, can vary significantly depending on the composition of the solvent. Understanding the factors that influence glucose solubility is essential for optimizing processes and ensuring accurate quantification of total soluble sugars in plant tissue extracts.
Understanding Glucose Solubility in Water
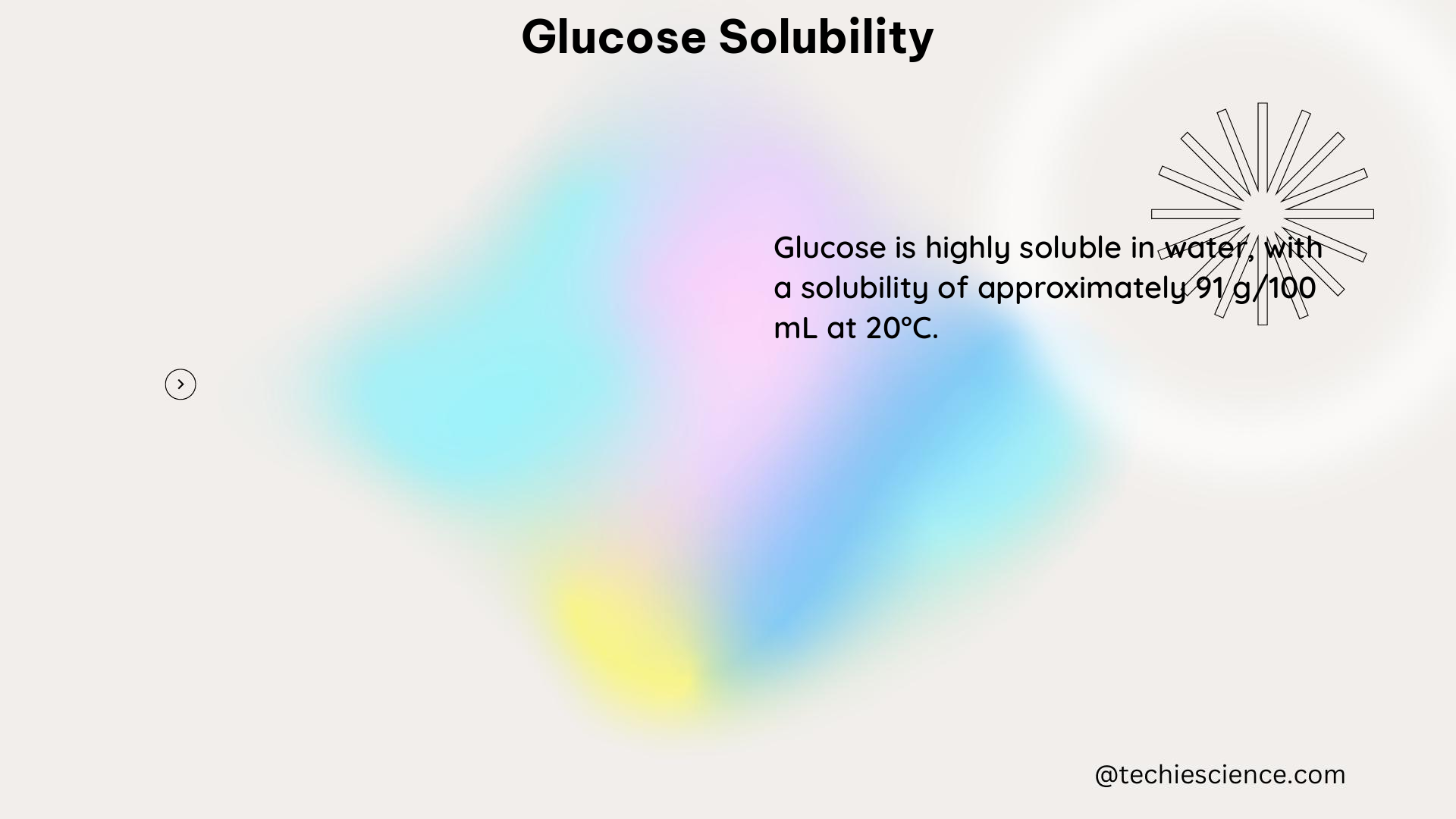
The solubility of glucose in water is approximately 900 g/L at 25°C, which means that 900 grams of glucose can be dissolved in one liter of water at this temperature. This high solubility is due to the formation of hydrogen bonds between the glucose molecules and the water molecules.
The solubility of glucose in water can be described by the following equation:
ΔG = ΔH - TΔS
Where:
– ΔG is the change in Gibbs free energy
– ΔH is the change in enthalpy
– T is the absolute temperature
– ΔS is the change in entropy
At 25°C, the dissolution of glucose in water is a spontaneous process, as the change in Gibbs free energy (ΔG) is negative, indicating a favorable thermodynamic driving force. The negative ΔG value is a result of the favorable enthalpy change (ΔH) due to the formation of hydrogen bonds, which outweighs the decrease in entropy (ΔS) associated with the ordering of the water molecules around the glucose molecules.
Factors Affecting Glucose Solubility in Water
Several factors can influence the solubility of glucose in water, including:
-
Temperature: The solubility of glucose in water increases with increasing temperature. This is because the higher kinetic energy of the molecules at higher temperatures helps to overcome the intermolecular attractions, allowing more glucose to dissolve.
-
Pressure: The solubility of glucose in water is relatively insensitive to changes in pressure, as the dissolution process does not involve a significant change in volume.
-
Molecular Structure: The specific molecular structure of glucose, with its multiple hydroxyl groups, facilitates the formation of hydrogen bonds with water molecules, enhancing its solubility.
-
Impurities: The presence of impurities in the water can affect the solubility of glucose by altering the intermolecular interactions and the thermodynamic driving force for dissolution.
Glucose Solubility in Ethanol-Water Mixtures
In ethanol-water mixtures, the solubility of glucose decreases as the ethanol concentration increases. At 25°C, the solubility of glucose in pure ethanol is only about 6.7 g/L, while in a 50:50 (v/v) mixture of ethanol and water, the solubility increases to approximately 250 g/L.
This behavior can be explained by the fact that glucose forms hydrogen bonds with water molecules, and the addition of ethanol disrupts these bonds, reducing the solubility of glucose. Ethanol, being a less polar solvent compared to water, is less effective at solvating the glucose molecules, leading to a decrease in solubility.
The solubility of glucose in ethanol-water mixtures can be described by the following equation:
ln(x_g) = A + B/(T) + C*ln(T) + D*x_e
Where:
– x_g is the mole fraction of glucose in the solution
– T is the absolute temperature
– x_e is the mole fraction of ethanol in the solution
– A, B, C, and D are empirical constants that depend on the specific system.
This equation allows for the prediction of glucose solubility in ethanol-water mixtures at different temperatures and ethanol concentrations.
Glucose Solubility in Ionic Liquid (IL) + Antisolvent Mixtures
In ionic liquid (IL) + antisolvent mixtures, the solubility of glucose can be significantly enhanced, making it possible to separate glucose from ILs. An extended study on the solubility of glucose in IL + antisolvent mixtures has been performed, and the results show that the solubility of glucose in these mixtures is highly dependent on the type of IL and antisolvent used.
The solubility of glucose in IL + antisolvent mixtures can be described by the following equation:
ln(x_g) = A + B/T + C*ln(T) + D*x_a
Where:
– x_g is the mole fraction of glucose in the solution
– T is the absolute temperature
– x_a is the mole fraction of the antisolvent in the mixture
– A, B, C, and D are empirical constants that depend on the specific IL and antisolvent system.
The choice of IL and antisolvent can significantly impact the solubility of glucose, as the interactions between the glucose, IL, and antisolvent molecules can vary greatly. Careful selection of the IL and antisolvent combination is crucial for optimizing the glucose solubility and separation process.
Quantifying Total Soluble Sugars in Plant Tissue Extracts
When quantifying total soluble sugars in plant tissue extracts, a standard curve is necessary for each day of measurement. The standard curve should be prepared using glucose solutions with concentrations ranging from 0 to 25 µg/well. The R^2 of the standard curve should be greater than 0.990, and the typical standard deviation between technical sample replicates should be between 0.10 and 0.30, depending on the precision of the multi-channel or repeat pipettor used.
The use of a standard curve is essential to ensure accurate and reproducible results, as it allows for the conversion of the measured absorbance values of the plant tissue extracts into glucose concentrations. The standard curve should be linear and have a high coefficient of determination (R^2) to ensure the reliability of the quantification.
It is important to note that the standard curve should be prepared fresh for each day of measurement, as the solubility of glucose can be affected by factors such as temperature, pH, and the presence of other compounds in the extract. Maintaining consistent experimental conditions and following standardized protocols is crucial for obtaining reliable and reproducible results.
Conclusion
Glucose solubility is a critical parameter that determines the maximum amount of glucose that can be dissolved in a given volume of solvent at a specific temperature. The solubility of glucose in water is remarkably high, making it an ideal candidate for various applications. However, the solubility of glucose in other solvents, such as ethanol-water mixtures and ionic liquid (IL) + antisolvent mixtures, can vary significantly depending on the composition of the solvent.
Understanding the factors that influence glucose solubility, such as temperature, pressure, molecular structure, and the presence of impurities, is essential for optimizing processes and ensuring accurate quantification of total soluble sugars in plant tissue extracts. The use of standard curves and adherence to standardized protocols are crucial for obtaining reliable and reproducible results when quantifying total soluble sugars in plant tissue extracts.
By delving into the science behind glucose solubility, researchers and scientists can gain valuable insights that can be applied to a wide range of industries, from food and pharmaceuticals to biofuel production and beyond.
References:
- Solubility of d Glucose in Water and Ethanol/Water Mixtures: https://www.researchgate.net/publication/244465396_Solubility_of_d_Glucose_in_Water_and_EthanolWater_Mixtures
- Lab: Solubility Assignment: Reflect on the Lab: https://quizlet.com/416074924/lab-solubility-assignment-reflect-on-the-lab-flash-cards/
- Standardized protocols and procedures can precisely and … – NCBI: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6301340/
- Solubility of Glucose in Ionic Liquid Plus Antisolvent Mixtures: https://www.researchgate.net/publication/231376998_Solubility_of_Glucose_in_Ionic_Liquid_Plus_Antisolvent_Mixtures
- Total Soluble Sugar Quantification from Ethanolic Plant Extracts: https://www.protocols.io/view/total-soluble-sugar-quantification-from-ethanolic-b2nsqdee.html

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.