Glass density is a fundamental property that plays a crucial role in various applications, from forensic analysis to material science. This comprehensive guide delves into the intricacies of glass density, providing a wealth of technical details and practical insights to help you navigate the complexities of this essential characteristic.
Understanding Glass Density: The Basics
Glass density is a measure of the mass per unit volume of a glass sample. It is a crucial property that varies with changes in the glass composition and thermal history. Glass density can be used as a screening technique to differentiate between glass samples, as two samples with different densities cannot have originated from the same source.
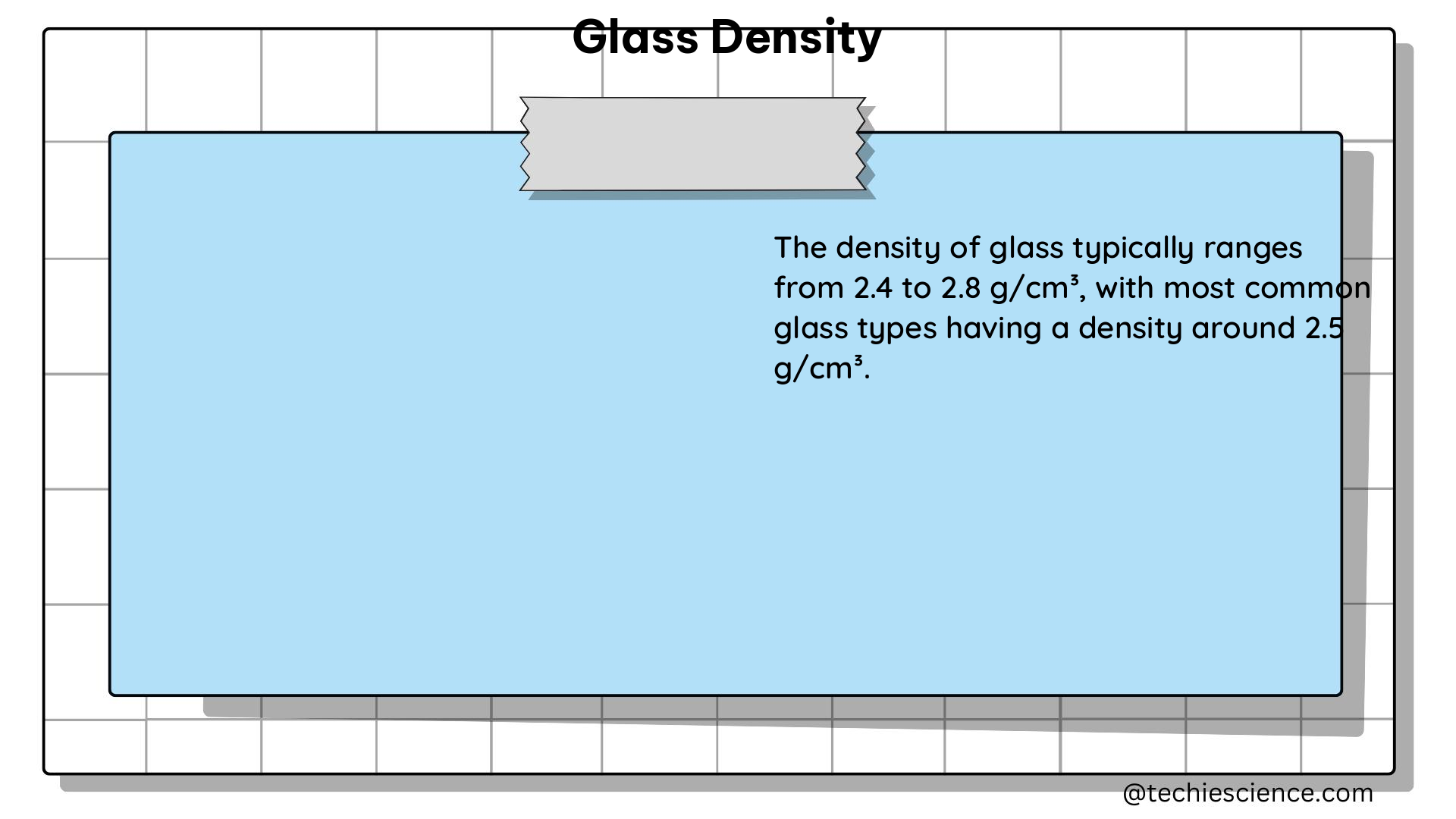
The density of glass is typically measured in grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). The specific density of glass can range from around 2.2 g/cm³ for soda-lime glass to 6.0 g/cm³ for lead crystal glass, depending on the chemical composition and manufacturing process.
Measuring Glass Density: Techniques and Methodologies
Accurately measuring the density of glass requires the use of specialized techniques and equipment. The most common methods for determining glass density include:
1. Density Gradient Columns
Density gradient columns are a widely used technique for measuring the density of glass samples. This method involves creating a liquid column with a linear density gradient, into which the glass sample is placed. The position of the sample within the column is then used to determine its density.
The density gradient column is typically prepared by mixing two liquids with different densities, such as water and a high-density liquid like bromoform or diiodomethane. The resulting column has a linear density gradient, with the highest density at the bottom and the lowest density at the top.
To use this method, the glass sample is carefully placed into the column and allowed to settle at the point where its density matches the surrounding liquid. The position of the sample within the column is then measured, and its density is determined by comparing the sample’s position to a calibration curve.
2. Analytical Balance and Plummet
The analytical balance and plummet method involves weighing a plummet (a small, dense object) immersed in a liquid solution and calculating the density of the liquid and the glass sample.
In this technique, the glass sample is first weighed in air to determine its mass. The sample is then suspended in a liquid solution, and the plummet is immersed in the same solution. The mass of the plummet is measured both in air and while submerged in the liquid. Using the known density of the plummet and the mass measurements, the density of the liquid and the glass sample can be calculated.
3. Density Meter
The density meter method uses a specialized instrument to directly measure the density of a liquid solution in which the glass sample is suspended. The density meter typically employs a vibrating U-shaped tube filled with the liquid-glass mixture, and the instrument measures the resonant frequency of the tube to determine the density of the solution.
To use this method, the glass sample is first weighed, and then the sample is placed in a liquid solution with a known density. The density of the liquid-glass mixture is then measured using the density meter, and the density of the glass sample can be calculated based on the known liquid density and the mass of the sample.
4. Sink/Float (Comparative) Method
The sink/float (comparative) method is a simple and inexpensive technique for measuring the density of glass samples. This method involves comparing the density of an unknown glass sample to that of a known reference sample by observing whether the unknown sample sinks or floats in a liquid solution.
To use this method, the unknown glass sample is placed in a liquid solution with a known density. If the unknown sample sinks, its density is greater than the liquid density; if it floats, its density is less than the liquid density. By comparing the behavior of the unknown sample to that of a known reference sample, the density of the unknown sample can be estimated.
Factors Affecting Glass Density Measurements
Accurate and reliable glass density measurements require careful attention to several factors, including:
-
Sample Preparation: Proper sample preparation is essential to ensure accurate density measurements. Glass samples should be free of inclusions, bubbles, or other impurities that could affect the density.
-
Temperature: Glass density is temperature-dependent, so measurements should be conducted at a consistent and controlled temperature to ensure accurate results.
-
Liquid Purity: The purity and composition of the liquid used in density measurements can significantly impact the results. Impurities or variations in the liquid’s density can lead to inaccurate glass density values.
-
Calibration and Standardization: Proper calibration of the measurement equipment and the use of standard reference materials are essential for ensuring the accuracy and precision of glass density measurements.
-
Sample Size and Shape: The size and shape of the glass sample can also affect the density measurement, as larger or irregularly shaped samples may be more challenging to suspend or immerse in the liquid solution.
Practical Applications of Glass Density Measurements
Glass density measurements have a wide range of practical applications, including:
-
Forensic Analysis: Glass density can be used as a screening technique to differentiate between glass samples, which can be valuable in forensic investigations.
-
Material Science: Glass density is an important property in the development and characterization of new glass compositions and manufacturing processes.
-
Quality Control: Monitoring the density of glass products can help ensure consistent quality and identify any variations in the manufacturing process.
-
Architectural and Engineering Applications: Glass density data is essential for calculating the structural loads and performance of glass components in buildings and other structures.
-
Recycling and Waste Management: Glass density can be used to separate and sort different types of glass for recycling and waste management purposes.
Numerical Examples and Data Points
To illustrate the practical application of glass density measurements, let’s consider the following examples:
-
Soda-Lime Glass Density: Soda-lime glass, the most common type of glass, typically has a density range of 2.4 to 2.6 g/cm³. For example, a sample of soda-lime glass with a measured density of 2.52 g/cm³ would be considered within the expected range.
-
Lead Crystal Glass Density: Lead crystal glass, known for its high refractive index and brilliance, has a higher density range of 2.9 to 4.0 g/cm³. A lead crystal glass sample with a measured density of 3.24 g/cm³ would be consistent with the expected values for this type of glass.
-
Borosilicate Glass Density: Borosilicate glass, commonly used in laboratory glassware and cookware, has a density range of 2.2 to 2.3 g/cm³. A borosilicate glass sample with a measured density of 2.23 g/cm³ would be within the typical range for this material.
-
Tempered Glass Density: Tempered glass, a type of safety glass, has a density range of 2.5 to 2.6 g/cm³. A tempered glass sample with a measured density of 2.54 g/cm³ would be considered normal for this type of glass.
-
Glass Fiber Density: Glass fibers, used in reinforced composites and insulation, have a density range of 2.5 to 2.6 g/cm³. A glass fiber sample with a measured density of 2.58 g/cm³ would be within the expected range for this material.
These examples illustrate the typical density ranges for various types of glass, which can be used as a reference when conducting glass density measurements and analysis.
Conclusion
Glass density is a critical property that plays a vital role in numerous applications, from forensic analysis to material science. This comprehensive guide has provided a detailed overview of the techniques and methodologies used to measure glass density, as well as the factors that can affect the accuracy and reliability of these measurements.
By understanding the intricacies of glass density, you can unlock a wealth of insights and applications in your field of study or research. Whether you’re a physics student, a materials scientist, or a forensic investigator, this guide has equipped you with the knowledge and tools necessary to master the complexities of glass density.
References:
- Density of glass – YouTube. (2011-01-03). Retrieved from https://www.youtube.com/watch?v=E2qvu5ugNXY
- Glass Density Determination. (July 2004). Retrieved from https://www.asteetrace.org/static/images/pdf/07%20Glass%20Density%20Determination.pdf
- dscompared to known values for different types of glass to see if the… (n.d.). Retrieved from https://stonehill-website.s3.amazonaws.com/files/resources/glass-density.doc

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.