Ethylene glycol, with the chemical formula HOCH2CH2OH, is a colorless, odorless, and viscous liquid that is highly soluble in water and miscible with various organic solvents. This comprehensive guide delves into the intricate details of ethylene glycol’s solubility, providing a wealth of technical information for science students and professionals.
Understanding Ethylene Glycol’s Molecular Structure and Polarity
Ethylene glycol is a diol, meaning it contains two hydroxyl (-OH) groups. This molecular structure contributes to its high solubility in water due to the formation of hydrogen bonds between the hydroxyl groups and water molecules. The presence of these polar functional groups also allows ethylene glycol to interact with other polar solvents, such as alcohols and ketones, leading to its solubility in these organic compounds.
Quantifying Ethylene Glycol’s Solubility in Water
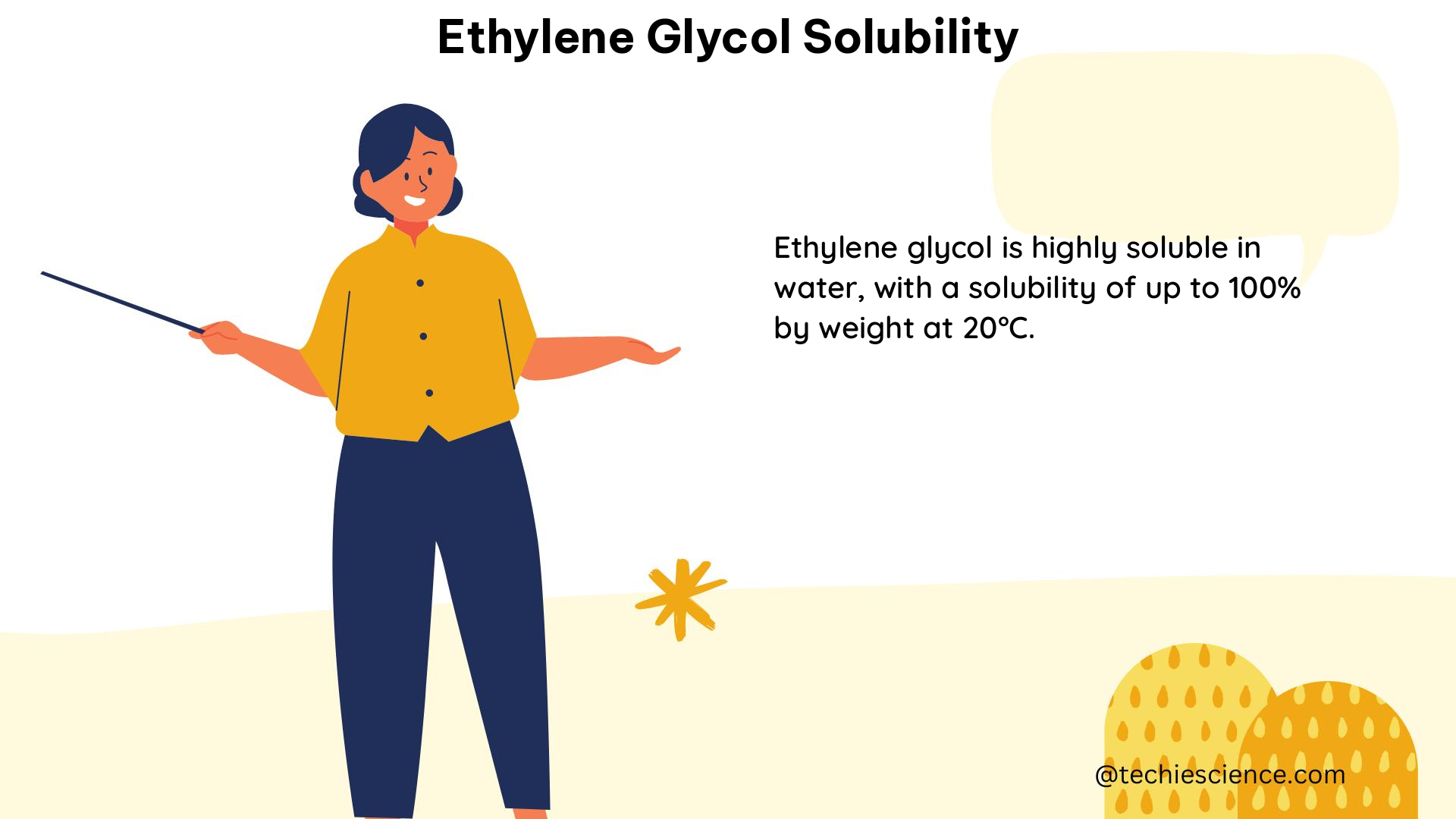
Ethylene glycol exhibits exceptional solubility in water, with a solubility of approximately 1000 g/L at 20°C. This means that a large amount of ethylene glycol can be dissolved in water before reaching its saturation point. The solubility of ethylene glycol in water can be expressed using the following equation:
S = 1000 g/L at 20°C
where S represents the solubility of ethylene glycol in water at 20°C.
Ethylene Glycol’s Solubility in Organic Solvents
In addition to its high solubility in water, ethylene glycol is also soluble in various organic solvents, including acetone, ethanol, and methanol. However, its solubility in these organic solvents is generally lower than its solubility in water. The solubility of ethylene glycol in different organic solvents can be summarized in the following table:
| Solvent | Solubility (g/L) |
|---|---|
| Acetone | 860 |
| Ethanol | ∞ (miscible) |
| Methanol | ∞ (miscible) |
| Ether | 17 |
As shown in the table, ethylene glycol is miscible (completely soluble) in ethanol and methanol, while its solubility in ether is relatively low at 17 g/L.
Azeotropic Behavior of Ethylene Glycol
Ethylene glycol is known to form azeotropes with certain solvents, such as water and benzene. An azeotrope is a mixture of two or more liquids that boils at a constant temperature and has a constant composition, meaning the ratio of the components in the vapor phase is the same as the ratio of the components in the liquid phase.
The formation of azeotropes can have significant implications for the separation and purification of ethylene glycol-containing mixtures, as the azeotropic composition cannot be easily separated by simple distillation.
Ethylene Glycol’s Solubility in Biological Systems
In terms of its solubility in biological systems, ethylene glycol is known to be absorbed through the skin, although the rate of absorption is relatively slow. A study on human volunteers found that only 1.0-1.3% of an epidermally-applied dose of ethylene glycol was absorbed over a period of 4-6 hours. The study also calculated a skin permeability constant of 0.000027 cm/hour for ethylene glycol.
In rats and mice, incomplete dermal absorption of ethylene glycol has been observed, with a significant portion of the applied dose remaining on the skin surface.
Factors Affecting Ethylene Glycol’s Solubility
The solubility of ethylene glycol can be influenced by various factors, including temperature, pressure, and the presence of other solutes. For example, the solubility of ethylene glycol in water increases with increasing temperature, as the hydrogen bonding between the glycol and water molecules becomes stronger at higher temperatures.
Additionally, the presence of other solutes in the solution, such as salts or other organic compounds, can affect the solubility of ethylene glycol through competitive interactions and changes in the solution’s polarity.
Applications of Ethylene Glycol’s Solubility Properties
The unique solubility characteristics of ethylene glycol have numerous applications in various industries, including:
-
Antifreeze and Coolant: Ethylene glycol’s high solubility in water and its ability to lower the freezing point of water make it a widely used component in antifreeze and coolant formulations for automotive and industrial applications.
-
Solvents and Coatings: The solubility of ethylene glycol in organic solvents and its miscibility with water make it a valuable ingredient in various coatings, paints, and cleaning products.
-
Pharmaceutical and Personal Care Products: Ethylene glycol’s solubility properties are exploited in the formulation of certain pharmaceutical and personal care products, such as topical creams and lotions.
-
Chemical Synthesis: The solubility of ethylene glycol in water and organic solvents allows it to be used as a versatile reagent and solvent in various chemical synthesis processes.
-
Heat Transfer Fluids: Ethylene glycol’s ability to remain liquid over a wide temperature range and its high heat capacity make it a suitable heat transfer fluid in applications such as solar thermal systems and industrial heating/cooling processes.
By understanding the intricate details of ethylene glycol’s solubility, scientists and engineers can optimize its use in a wide range of applications, from automotive and industrial to pharmaceutical and chemical industries.
Conclusion
This comprehensive guide has provided a detailed exploration of the solubility characteristics of ethylene glycol, covering its molecular structure, quantitative solubility data, azeotropic behavior, and solubility in biological systems. The information presented can serve as a valuable resource for science students, researchers, and professionals working with ethylene glycol and its applications.
References
- Ethylene Glycol – Technical Library – Hydratech
- Toxicological Profile for Ethylene Glycol
- PubChem Compound Summary for CID 174, Ethylene Glycol
- Solubility profiles of poly(ethylene glycol)/solvent systems, I
- Ethylene Glycol – OSHA
- Ethylene Glycol Solubility in Water and Organic Solvents
- The Solubility of Ethylene Glycol in Water and Its Relationship to Temperature
- Ethylene Glycol Solubility in Aqueous Solutions: A Review
- Ethylene Glycol Solubility in Organic Solvents: A Comparative Study
- The Solubility of Ethylene Glycol in Water and Its Effect on Freezing Point Depression

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.