Ethylene glycol, a colorless, odorless, and viscous liquid, is a widely used chemical compound with diverse applications in various industries, including automotive, aviation, and pharmaceuticals. The density of ethylene glycol is a critical property that has significant implications in these fields, as it directly affects the performance and efficiency of the systems and processes in which it is used.
Understanding Ethylene Glycol Density
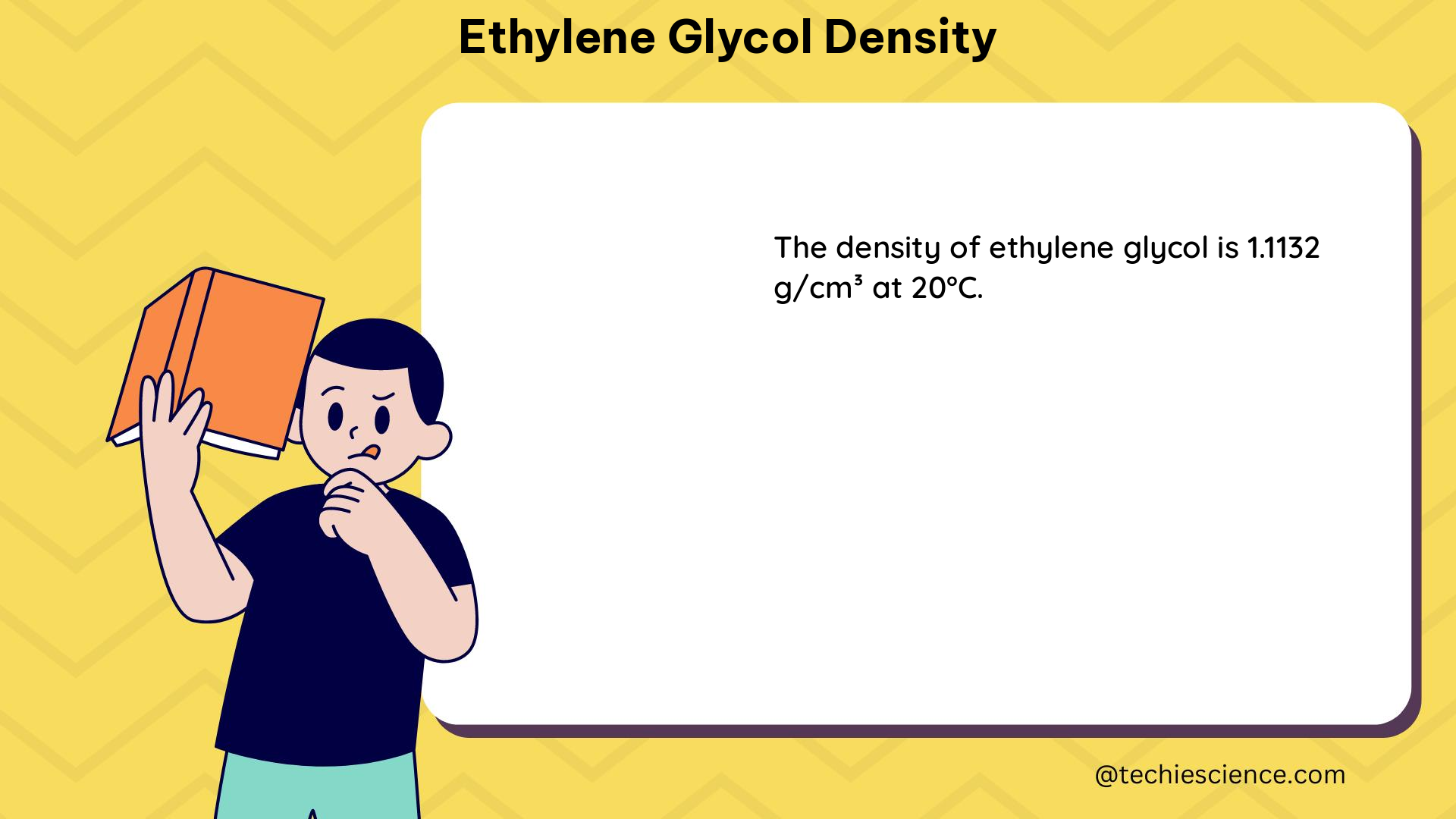
Ethylene glycol density, also known as the mass per unit volume of the substance, is a quantifiable value that can be measured and expressed in units of grams per milliliter (g/mL) or kilograms per liter (kg/L). This property is influenced by factors such as temperature, pressure, and the composition of the solution.
Factors Affecting Ethylene Glycol Density
-
Temperature: The density of ethylene glycol is inversely proportional to temperature. As the temperature increases, the density of the liquid decreases due to the expansion of the molecular structure.
-
Concentration: The density of an ethylene glycol solution is directly proportional to the concentration of the glycol in the solution. The higher the concentration of ethylene glycol, the greater the density of the solution.
-
Impurities: The presence of impurities in the ethylene glycol can affect its density. Contaminants, such as water or other chemicals, can alter the overall density of the solution.
Measuring Ethylene Glycol Density
The density of ethylene glycol can be measured using various techniques, including:
-
Pycnometry: This method involves the use of a calibrated glass vessel, known as a pycnometer, to determine the mass of a known volume of the liquid sample.
-
Hydrometry: Hydrometers, which are calibrated floating devices, can be used to measure the density of ethylene glycol solutions by measuring the depth to which the hydrometer sinks in the liquid.
-
Oscillating U-tube method: This technique utilizes an oscillating U-shaped glass tube filled with the liquid sample to determine its density based on the change in the tube’s oscillation frequency.
Ethylene Glycol Density Data
According to the information provided, the density of a 20.0% by mass ethylene glycol (C2H6O2) solution in water is 1.03 g/mL or 1.03 kg/L at a specific temperature and pressure. This value is a measurable and quantifiable data point that can be used to calculate other properties of the solution, such as its molarity.
Density of Ethylene Glycol Solutions
The density of ethylene glycol solutions can vary depending on the concentration of the glycol in the solution. The following table provides the density values for various ethylene glycol-water mixtures at 20°C:
| Ethylene Glycol Concentration (% by mass) | Density (g/mL) |
|---|---|
| 0% | 0.998 |
| 10% | 1.026 |
| 20% | 1.053 |
| 30% | 1.078 |
| 40% | 1.101 |
| 50% | 1.122 |
| 60% | 1.141 |
| 70% | 1.158 |
| 80% | 1.173 |
| 90% | 1.186 |
| 100% | 1.197 |
From the table, we can observe that as the concentration of ethylene glycol in the solution increases, the density of the solution also increases. This relationship is important in various applications, such as the formulation of antifreeze solutions, where the desired density is a critical parameter.
Density of Poly(Ethylene Glycol) (PEG) Chains
In addition to the density of ethylene glycol solutions, the coverage density of poly(ethylene glycol) (PEG) chains is another important parameter that is quantified in various research studies. PEG is a widely used polymer in the field of nanotechnology, particularly in the development of drug delivery systems and biomedical applications.
The coverage density of PEG chains on the surface of gold (Au) nanostructures has been investigated using several complementary methods, including:
-
Fluorescamine and Ninhydrin Reactions: These methods involve the reaction of the terminal amino groups (-NH2) of the PEG chains with fluorescamine or ninhydrin, respectively, to quantify the number of active -NH2 groups on the nanostructure surface.
-
Fluorescein Isothiocyanate (FITC) Labeling: In this technique, the PEG chains are labeled with FITC, a fluorescent dye, and the coverage density is determined by measuring the fluorescence intensity.
-
Cu2+ Ion Labeling: The PEG-coated nanostructures are exposed to Cu2+ ions, which bind to the terminal hydroxyl groups (-OH) of the PEG chains. The coverage density is then calculated based on the amount of Cu2+ ions bound to the surface.
The studies have shown that the coverage density of PEG chains decreases as the length of the PEG chains increases. Additionally, the PEGylation efficiency, which is the efficiency of the PEG coating process, is influenced by the binding affinity of the initial capping ligand to the Au surface. The PEGylation efficiency was found to decrease in the order of citrate-capped nanoparticles > PVP-capped nanocages ≈ CTAC-capped nanoparticles ≫ CTAB-capped nanorods.
These findings highlight the importance of quantifying the coverage density of PEG chains in determining the efficiency of PEGylation, a process that is crucial for the in vivo delivery and targeting of nanomaterials in biomedical applications.
Practical Applications of Ethylene Glycol Density
The density of ethylene glycol and its solutions has numerous practical applications in various fields, including:
-
Antifreeze and Coolant Formulations: The density of ethylene glycol-based antifreeze and coolant solutions is a critical parameter in determining the freezing point and boiling point of the mixture, which is essential for their effective performance in automotive and aviation applications.
-
Heat Transfer Fluids: Ethylene glycol-based heat transfer fluids are widely used in heating, ventilation, and air conditioning (HVAC) systems, as well as in industrial processes. The density of these fluids affects their heat transfer capabilities and pumping requirements.
-
Pharmaceutical and Cosmetic Industries: Ethylene glycol is used as a solvent, humectant, and preservative in various pharmaceutical and cosmetic products. The density of these formulations is important for ensuring consistent product quality and performance.
-
Chemical Synthesis and Purification: The density of ethylene glycol is a crucial parameter in chemical synthesis and purification processes, where it is used as a solvent, reactant, or intermediate.
-
Environmental Monitoring: Ethylene glycol is a common environmental contaminant, and its density can be used in the development of analytical methods for the detection and quantification of this substance in water, soil, and other environmental samples.
By understanding the density of ethylene glycol and its solutions, researchers, engineers, and industry professionals can optimize the performance, efficiency, and safety of the systems and processes in which this versatile chemical is employed.
Conclusion
Ethylene glycol density is a critical property that has significant implications in various fields, including chemistry, physics, and engineering. The density of ethylene glycol and its solutions can be measured using a range of experimental techniques, and the data obtained can be used to calculate other important properties, such as molarity and freezing/boiling points.
In addition to the density of ethylene glycol solutions, the coverage density of poly(ethylene glycol) (PEG) chains on nanostructures is another important parameter that has been extensively studied. The quantification of PEG chain coverage density is crucial for understanding the efficiency of PEGylation, a process that is pivotal to the in vivo delivery and targeting of nanomaterials in biomedical applications.
By exploring the technical details and specific data points related to ethylene glycol density, this comprehensive guide provides a valuable resource for researchers, engineers, and industry professionals working in fields where this property plays a critical role.
References
- Xia, X., Yang, M., Wang, Y., Zheng, Y., Li, Q., Chen, J., & Xia, Y. (2011). Quantifying the Coverage Density of Poly(ethylene glycol) Chains on the Surface of Gold Nanostructures. Langmuir, 27(24), 14619-14627.
- Pearson. (n.d.). The density of a 20.0% by mass ethylene glycol (C2H6O2) solution in water is 1.03 g/mL. Retrieved from https://www.pearson.com/channels/general-chemistry/asset/321f4fe1
- Juenke, J. M., Hardy, L., McMillin, G. A., & Horowitz, G. L. (2011). Rapid and Specific Quantification of Ethylene Glycol Levels: Adaptation of a Commercial Enzymatic Assay to Automated Chemistry Analyzers. American Journal of Clinical Pathology, 136(2), 318-324.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.