Ethanol is a chemical compound that is commonly used as a solvent, fuel, and in the production of alcoholic beverages. Its molecular formula is C2H5OH, and it consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure of ethanol shows the arrangement of these atoms and their valence electrons. In this structure, the carbon atoms are bonded to each other by a single bond, and each carbon atom is also bonded to three hydrogen atoms. The oxygen atom is bonded to one of the carbon atoms by a single bond and also has two lone pairs of electrons. This Lewis dot structure helps us understand the bonding and electron distribution in ethanol.
Key Takeaways
| Atom | Number of Valence Electrons |
|---|---|
| Carbon | 4 |
| Hydrogen | 1 |
| Oxygen | 6 |
Understanding Lewis Dot Structure
Lewis dot structure, also known as electron dot structure or Lewis structure, is a visual representation of the arrangement of atoms and valence electrons in a molecule. It provides valuable insights into the chemical bonding and molecular geometry of a compound. Understanding Lewis dot structure is essential in the field of organic chemistry and plays a crucial role in predicting the behavior and properties of molecules.
Octet Rule
The Octet Rule is a fundamental concept in Lewis dot structure. It states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with a full outer shell of eight electrons. This rule applies to most elements, except for hydrogen, which only requires two electrons to achieve stability.
Steps for Determining Lewis Dot Structure
To determine the Lewis dot structure of a molecule, follow these steps:
- Calculate the total number of valence electrons by summing up the valence electrons of all the atoms in the molecule.
- Identify the central atom, which is usually the least electronegative element or the one with the highest valence.
- Connect the central atom to the surrounding atoms using single bonds.
- Distribute the remaining electrons as lone pairs around the atoms, starting with the outer atoms.
- If there are not enough electrons to satisfy the octet rule for all atoms, form multiple bonds by converting lone pairs into bonding pairs.
- Check if all atoms have achieved an octet or duet (in the case of hydrogen). If not, rearrange the electrons to form multiple bonds or expand the octet of the central atom if necessary.
Example: Ethanol (C2H6O)
Let’s apply the steps for determining Lewis dot structure to the ethanol molecule (C2H6O). Ethanol is an organic compound commonly found in alcoholic beverages.
- Calculate the total number of valence electrons:
- Carbon (C): 4 valence electrons x 2 = 8 electrons
- Hydrogen (H): 1 valence electron x 6 = 6 electrons
-
Oxygen (O): 6 valence electrons x 1 = 6 electrons
Total = 20 valence electrons -
Identify the central atom. In ethanol, carbon (C) is the central atom.
-
Connect the central atom to the surrounding atoms using single bonds:
- C – C
- C – H
- C – H
-
C – O
-
Distribute the remaining electrons as lone pairs around the atoms:
- Carbon (C): 4 electrons (2 lone pairs)
- Hydrogen (H): 0 electrons (no lone pairs)
-
Oxygen (O): 4 electrons (2 lone pairs)
-
Check if all atoms have achieved an octet or duet:
- Carbon (C): 8 electrons (satisfied octet)
- Hydrogen (H): 2 electrons (satisfied duet)
- Oxygen (O): 8 electrons (satisfied octet)
The Lewis dot structure of ethanol (C2H6O) consists of a central carbon atom bonded to two hydrogen atoms, one oxygen atom, and two lone pairs of electrons on the oxygen atom.
By understanding Lewis dot structure, we can visualize the arrangement of atoms and electrons in a molecule, predict the bond angles and lengths, and represent the chemical structure in a concise and informative manner. It serves as a foundation for further concepts in organic chemistry, such as resonance structures, hybridization, and the VSEPR theory for molecular geometry. Molecular models based on Lewis dot structures are widely used in chemistry education to enhance understanding and facilitate learning.
Detailed Study of Ethanol Lewis Dot Structure
Ethanol is a chemical compound with the molecular formula C2H5OH. It is commonly known as alcohol and is widely used as a solvent, fuel, and in the production of alcoholic beverages. Understanding the Lewis dot structure of ethanol is crucial in comprehending its chemical properties and behavior.
Ethanol Electron Dot Structure
The electron dot structure, also known as the Lewis structure, is a visual representation of the arrangement of atoms and valence electrons in a molecule. It provides insights into the chemical bonding and molecular geometry of a compound. In the case of ethanol, the Lewis dot structure reveals the sharing of electrons between carbon, hydrogen, and oxygen atoms.
Detailed Explanation of the Electron Distribution in Ethanol
In ethanol, the carbon atom (C) forms four covalent bonds, one with each of the two hydrogen atoms (H) and two with the oxygen atom (O). The oxygen atom, in turn, forms two covalent bonds, one with carbon and the other with hydrogen. The remaining hydrogen atom forms a single covalent bond with carbon. This sharing of electrons allows each atom to achieve a stable electron configuration.
Sharing of Electrons between Carbon, Hydrogen, and Oxygen Atoms
Let’s break down the Lewis dot structure of ethanol step-by-step:
-
Carbon (C): Carbon has four valence electrons. It forms four covalent bonds, one with each hydrogen atom and two with the oxygen atom. This allows carbon to achieve a full octet of electrons.
-
Hydrogen (H): Hydrogen has one valence electron. Each hydrogen atom forms a single covalent bond with carbon, contributing its electron to the shared pair.
-
Oxygen (O): Oxygen has six valence electrons. It forms two covalent bonds, one with carbon and the other with hydrogen. This allows oxygen to achieve a full octet of electrons.
Role of Carbon, Hydrogen, and Oxygen Atoms in Forming Bonds
The carbon atom in ethanol acts as the central atom, forming bonds with both hydrogen and oxygen. Hydrogen atoms contribute their single valence electron to form a bond with carbon, while oxygen atoms contribute two electrons to form a bond with carbon. This sharing of electrons allows all atoms to achieve a stable electron configuration and form covalent bonds.
Visual Representation of the Lewis Dot Structure for Ethanol
The Lewis dot structure for ethanol can be represented as follows:
H H
| |
C - C - O - H
| |
H H
In this diagram, the lines represent covalent bonds, and the dots represent valence electrons. The carbon atom is in the center, surrounded by hydrogen and oxygen atoms.
Description of the Lewis Dot Diagram for Ethanol
The Lewis dot diagram for ethanol shows the arrangement of atoms and the distribution of valence electrons. It provides a visual representation of how the atoms are connected and how electrons are shared between them. This diagram helps in understanding the molecular structure and bonding in ethanol.
Overall, studying the Lewis dot structure of ethanol enhances our understanding of its chemical properties and behavior. It allows us to analyze the arrangement of atoms, the sharing of electrons, and the formation of covalent bonds. This knowledge is essential in the field of organic chemistry and contributes to our understanding of molecular models and the principles of chemical structure.
Ethanol as a Lewis Acid
Discussion on Whether Ethanol Can Act as a Lewis Acid
Ethanol, with the chemical formula C2H5OH, is a commonly known alcohol that is widely used in various industries and as a recreational beverage. In organic chemistry, ethanol is often studied for its unique properties and its ability to participate in different chemical reactions. One interesting aspect of ethanol is its potential to act as a Lewis acid, which we will explore in this discussion.
To understand whether ethanol can act as a Lewis acid, let’s first delve into the concept of Lewis structures and chemical bonding. Lewis structures are diagrams that represent the arrangement of atoms and valence electrons in a molecule. They provide insight into the molecular geometry and the distribution of electrons within a compound.
The Lewis structure of the ethanol molecule consists of two carbon atoms (C), six hydrogen atoms (H), and one oxygen atom (O). The carbon atoms are bonded to each other through a single covalent bond, and each carbon atom is also bonded to three hydrogen atoms. The oxygen atom is bonded to one of the carbon atoms through a single covalent bond and has two lone pairs of electrons.
The resonance structures of ethanol further illustrate its chemical structure. The structural formula of ethanol shows that the oxygen atom can donate its lone pairs to form a double bond with one of the carbon atoms, resulting in resonance structures. This ability to donate electrons makes ethanol a potential Lewis acid.
In terms of hybridization, the carbon atoms in ethanol undergo sp3 hybridization, which allows for the formation of sigma bonds with other atoms. The VSEPR theory (Valence Shell Electron Pair Repulsion theory) predicts the molecular geometry of ethanol to be tetrahedral, with bond angles of approximately 109.5 degrees.
When considering the Lewis acid behavior of ethanol, it is important to note that Lewis acids are electron acceptors. They have an electron-deficient atom that can accept a lone pair of electrons from a Lewis base. In the case of ethanol, the carbon atom bonded to the oxygen atom can act as the electron-deficient center, attracting electron pairs from a Lewis base.
However, while ethanol has the potential to act as a Lewis acid, it is not as strong of a Lewis acid compared to other compounds. This is because the oxygen atom in ethanol is already involved in a covalent bond with the carbon atom, which reduces its electron-deficient nature. Additionally, the presence of the lone pairs on the oxygen atom can hinder its ability to accept additional electrons.
In conclusion, while ethanol has the potential to act as a Lewis acid due to its structural characteristics, it is not considered a strong Lewis acid. Its ability to accept electrons is limited by the presence of covalent bonds and lone pairs on the oxygen atom. Understanding the Lewis acid behavior of ethanol provides valuable insights into the field of organic chemistry and contributes to the broader knowledge of chemical bonding and molecular structures.
Structure of Ethanol
Overview of the Molecular Structure of Ethanol
Ethanol, also known as ethyl alcohol, is a chemical compound with the molecular formula C2H5OH. It is a colorless liquid that is commonly used as a solvent, fuel, and in the production of alcoholic beverages. Understanding the structure of ethanol is essential in the field of organic chemistry.
The Lewis structure of ethanol represents the arrangement of atoms and valence electrons in the molecule. It consists of two carbon atoms (C), six hydrogen atoms (H), and one oxygen atom (O). The carbon atoms are bonded together by a single covalent bond, and each carbon atom is also bonded to three hydrogen atoms. The oxygen atom is bonded to one of the carbon atoms and has two lone pairs of electrons.
To represent the structure of ethanol in a simplified manner, an electron dot diagram can be used. In this diagram, each atom is represented by its chemical symbol, and the valence electrons are represented by dots around the symbol. The structure of ethanol can be represented as follows:
- Carbon (C): C
- Hydrogen (H): H
- Oxygen (O): O
The structural formula of ethanol provides a more detailed representation of the arrangement of atoms and bonds in the molecule. It shows the specific bonds between the carbon, hydrogen, and oxygen atoms. The structural formula of ethanol is C2H5OH.
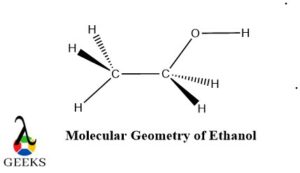
Geometry, Bond Length, and Bond Angle of Ethanol
The geometry of ethanol can be determined using the VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts the shape of molecules based on the repulsion between electron pairs. In the case of ethanol, the oxygen atom has two lone pairs of electrons, which repel the bonding pairs of electrons. As a result, the molecular geometry of ethanol is bent or V-shaped.
The bond length in ethanol refers to the distance between the nuclei of the bonded atoms. In ethanol, the carbon-oxygen bond length is approximately 1.43 angstroms, while the carbon-hydrogen bond length is approximately 1.09 angstroms. These bond lengths are determined by the strength of the covalent bonds between the atoms.
The bond angle in ethanol refers to the angle formed between two adjacent bonds. In ethanol, the carbon-oxygen-hydrogen bond angle is approximately 109.5 degrees. This angle is determined by the arrangement of the atoms and the repulsion between electron pairs.
Understanding the molecular structure of ethanol is crucial in various fields, including chemistry education and research. It allows scientists to study the properties and behavior of ethanol, as well as its interactions with other substances. By analyzing the structure, bond lengths, and bond angles, researchers can gain insights into the chemical properties and reactivity of ethanol.
In conclusion, the structure of ethanol, with its carbon, hydrogen, and oxygen atoms, plays a significant role in its chemical properties and behavior. The arrangement of atoms, bond lengths, and bond angles determine the shape and reactivity of the molecule, making ethanol a versatile compound in various applications.
Frequently Asked Questions
Q: Why is Lewis Dot Structure Important?
The Lewis dot structure is important because it helps us understand the chemical bonding and molecular geometry of a compound. It provides a visual representation of how atoms are connected and the arrangement of valence electrons. By using Lewis dot structures, we can determine the number of bonds, lone pairs, and the overall shape of a molecule.
Q: What is the Electron Dot Structure of Ethanol?
The electron dot structure of ethanol (C2H5OH) represents the arrangement of atoms and valence electrons in the molecule. In ethanol, there are two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure of ethanol shows the bonds between the atoms and the lone pairs of electrons on the oxygen atom.
Q: What is the Lewis Dot Structure of Ethanol (CH3CH2OH)?

The Lewis dot structure of ethanol (CH3CH2OH) is a representation of how the atoms are connected and the distribution of valence electrons in the molecule. In ethanol, there are two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure shows the bonds between the atoms and the lone pairs of electrons on the oxygen atom.
Q: What is the Lewis Dot Diagram for Ethanol?
The Lewis dot diagram for ethanol is a visual representation of the arrangement of atoms and valence electrons in the molecule. It shows the bonds between the carbon and hydrogen atoms, as well as the lone pairs of electrons on the oxygen atom. The Lewis dot diagram helps us understand the structure and bonding in ethanol.
Q: Is Ethanol a Lewis Acid?
No, ethanol (C2H5OH) is not a Lewis acid. A Lewis acid is a substance that can accept a pair of electrons. Ethanol does not have the ability to accept electrons, so it is not classified as a Lewis acid. Instead, ethanol is considered an organic compound and a common alcohol used in various applications.
Q: How Do You Find the Lewis Dot Structure?
To find the Lewis dot structure of a molecule, you need to follow a few steps. First, determine the total number of valence electrons by adding up the valence electrons of each atom in the molecule. Next, identify the central atom and connect it to the surrounding atoms with single bonds. Distribute the remaining electrons as lone pairs and multiple bonds to satisfy the octet rule for each atom. Finally, check if the Lewis dot structure is consistent with the molecular formula and the overall charge of the molecule.
Q: What is the Structure of Ethanol?
The structure of ethanol (C2H5OH) consists of two carbon atoms bonded to each other, with five hydrogen atoms attached to one carbon atom and one hydrogen atom attached to the other carbon atom. Additionally, there is an oxygen atom bonded to one of the carbon atoms. This arrangement of atoms and bonds is known as the structural formula of ethanol. It is important to note that the structure of ethanol can also be represented using a Lewis dot structure or a molecular model.
Conclusion
In conclusion, the Lewis dot structure of ethanol provides us with a visual representation of the arrangement of atoms and electrons in the molecule. By following a few simple rules, we can determine the number of valence electrons for each atom and distribute them accordingly. In the case of ethanol, we can see that it consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure helps us understand the bonding and electron distribution within the molecule, which is crucial for understanding its chemical properties and reactions. Overall, the Lewis dot structure is a valuable tool in the study of organic chemistry.
Frequently Asked Questions
What is the Lewis dot structure?
The Lewis dot structure, also known as electron dot structure, is a graphical representation of the molecular structure of a chemical compound. It shows the arrangement of valence electrons around the atoms in the molecule, indicating how they are involved in chemical bonding. The structure helps in understanding the type of bonding (covalent or ionic), the number of bond pairs, and lone pairs associated with each atom.
How can I find the Lewis dot structure of a molecule?
To find the Lewis dot structure of a molecule, follow these steps:
- Identify the total number of valence electrons in the molecule.
- Draw a skeleton structure of the molecule where the least electronegative atom is usually the central atom.
- Distribute the electrons among the atoms, starting with the outer atoms, following the octet rule.
- If any atoms lack an octet, form double or triple bonds as necessary.
- Check for resonance structures if applicable.
Is ethanol a Lewis acid?
No, ethanol is not a Lewis acid. In Lewis theory, a Lewis acid is a species that can accept an electron pair. Ethanol, with the molecular formula C2H5OH, is a neutral molecule and does not have a vacant orbital to accept an electron pair, so it does not act as a Lewis acid.
What is the structure of ethanol?
The ethanol molecule, also known as ethyl alcohol, has the molecular formula C2H5OH. Its structure consists of two carbon atoms bonded together, with one bonded to an -OH (hydroxyl) group, making it an alcohol. The remaining bonds of the carbon atoms are filled with hydrogen atoms. The carbon-oxygen bond is polar, and the molecule has a bent molecular geometry around the oxygen atom due to the presence of a lone pair of electrons.
How to deactivate logical volume in Linux?
This question is unrelated to the terms provided. However, to deactivate a logical volume in Linux, you can use the lvchange command with the -an option followed by the name of the volume. For example: lvchange -an /dev/myvg/mylv. Please ensure to replace ‘myvg’ and ‘mylv’ with your volume group and logical volume names respectively.
What is the Lewis dot structure of ethyl alcohol?
The Lewis dot structure of ethyl alcohol (ethanol) shows that the two carbon atoms are bonded together, with one carbon atom also bonded to an oxygen atom, which is further bonded to a hydrogen atom, forming a hydroxyl (-OH) group. The remaining bonds of the carbon atoms are filled with hydrogen atoms. The oxygen atom has two lone pairs of electrons.
Why is the Lewis dot structure important?
The Lewis dot structure is important because it provides a simple way to visualize the arrangement of valence electrons in a molecule. It helps in understanding the type of bonding (covalent or ionic), the number of bond pairs, and lone pairs associated with each atom. This information is crucial for predicting the molecule’s properties, such as its reactivity, polarity, and phase of matter.
What is the Lewis dot structure of LiBr?
The Lewis dot structure of lithium bromide (LiBr) shows that lithium (Li) donates one electron to bromine (Br), forming an ionic bond. Lithium becomes a positive ion (Li+) and bromine becomes a negative ion (Br-). This is due to the difference in electronegativity between the two atoms.
What is the Lewis dot structure of ethanol?
The Lewis dot structure of ethanol (C2H5OH) shows that the two carbon atoms are bonded together, with one carbon atom also bonded to an oxygen atom, which is further bonded to a hydrogen atom, forming a hydroxyl (-OH) group. The remaining bonds of the carbon atoms are filled with hydrogen atoms. The oxygen atom has two lone pairs of electrons.
What is the Lewis dot structure for ethanol (CH3CH2OH)?
The Lewis dot structure for ethanol (CH3CH2OH) shows that the two carbon atoms are bonded together, with one carbon atom also bonded to an oxygen atom, which is further bonded to a hydrogen atom, forming a hydroxyl (-OH) group. The remaining bonds of the carbon atoms are filled with hydrogen atoms. The oxygen atom has two lone pairs of electrons.

Hello, I am Mansi Sharma, I have completed my master’s in Chemistry. I personally believe that learning is more enthusiastic when learnt with creativity. I am a Subject Matter Expert in Chemistry.
Let’s connect through LinkedIn: https://www.linkedin.com/in/mansi-sharma22