Electron configuration of a periodic element is found to express the electron arrangement in the atomic orbitals. Let us draw attention on electron configuration of Erbium below:
Erbium is an artificially isolated metal comes in solid state. The metal has 68 atomic number. It is classified in periodic table as a Lanthanide element. With high melting and boiling point it is found to be slightly electronegative in the periodic table.
Erbium electron configuration would be described by highlighting the rules followed in representing the configuration as well as orbital diagrams and other of the element throughout this article.
How to write erbium electron configuration
The step by step approach of writing electron configuration is given below:
- Step 1: Writing the shell number is the fundamental step.
- It can be noticed that Erbium has total 6 electron shell
- Step 2: Indicating the orbitals is followed as the next step.
- There are four orbitals which hold the electrons in an atom those are s, p, d and f.
- s orbitals have highest capacity of holding 2 electrons and p, d and f have capacity if holding 6, 10 and 14 electrons respectively.
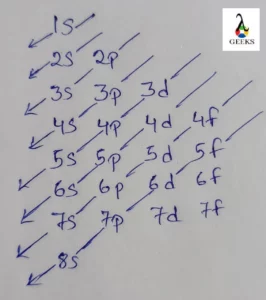
- The orbitals are written in a manner of 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s….
- Step 3: Final representation by putting subscripts is the final step for writing electron configuration.
- Finally the number of electrons is put in the orbitals as per their highest capacity in the form of subscripts.
- The manner of writing the final configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f12 5s2 5p6 6s2.
Erbium electron configuration diagram
According to Aufbau principle, the electron configuration of Erbium can be represented in the following manner:
Erbium electron configuration notation
The notations of electron configuration is as follows:
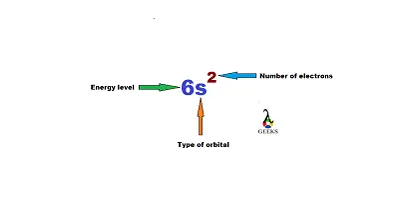
[Xe] 4f12 6s2
The above notation is depicting that in the 4th shell and f orbital there is 12 electron in Erbium atom. In 6th shell and s orbital there is 2 electrons in Er. The numbers of shells are noted by general numbers. Alphabets, s, p, d and f are orbital notations. Subscripts are used to denote electron numbers.
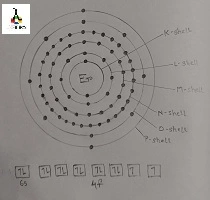
Ground state Erbium electron configuration
The Ground state Erbium electron configuration is [Xe] 4f12 6s2. Here the symbol of the element also changes to 3H6.
Excited state of Erbium electron configuration
The electron configuration of Erbium in exited state is considered to be [Xe] 4f12 6s1 as in excited the last valence electron gets higher orbital position due to higher energy level gained by the atom.
Ground state Erbium orbital diagram
In ground state the orbital diagram of Erbium is found to possess filled s, p and d orbitals but the f orbital holds only 12 electrons where it has capacity of holding 14 orbitals. Here the filled orbitals are found to possess each electron in both clockwise and anticlockwise spinning model with respect to each other.
Conclusion
This article is concluding that Erbium is consuming total 68 electrons. Here the clear notation of orbital state of an Erbium atom is notifying that it has filled orbitals except f orbital. Aufbau principle has been identified as most reliable one for developing the electron arrangement structure inside the atom.
Also Read:
- Iodine electron configuration
- Oganesson electron configuration
- Nickel electron configuration
- Helium electron configuration
- Potassium electron configuration
- Radon electron configuration
- Rubidium electron configuration
- Scandium electron configuration
- Gadolinium electron configuration
- Neon electron configuration

Hi…..I am Sarnali Mukherjee, a graduate from the University of Calcutta. I love to teach and share knowledge on chemistry. I have gradually gained interest in article writing since one year ago. I would love to acquire more knowledge on my subject in the future.
Let’s connect through LinkedIn: