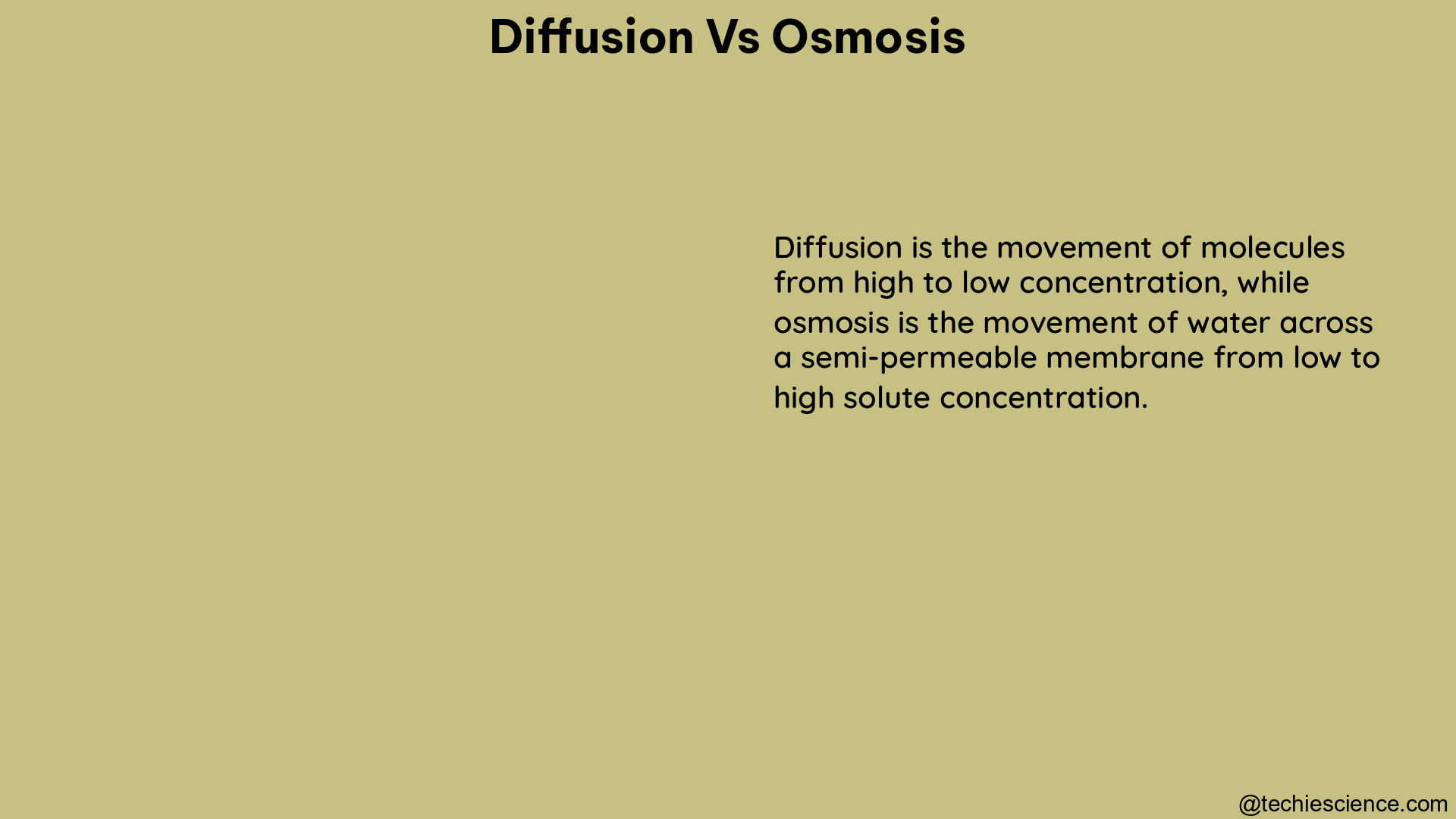
Diffusion and osmosis are two fundamental processes in biology that involve the movement of particles across a membrane. While both processes share the common principle of moving particles from an area of higher concentration to an area of lower concentration, they differ in the specific particles involved and the type of membranes through which they occur.
Understanding Diffusion
Diffusion is the process of particles moving from an area of higher concentration to an area of lower concentration, regardless of the type of particles or the membrane involved. This process is driven by the random motion of particles and continues until equilibrium is reached, meaning that the concentration of particles is equal on both sides of the membrane. Diffusion can occur through any type of membrane, including non-selective membranes that allow all types of particles to pass through.
Factors Affecting Diffusion
The rate of diffusion is influenced by several factors:
- Concentration Gradient: The greater the difference in concentration between the two areas, the faster the rate of diffusion.
- Membrane Permeability: The ease with which a substance can pass through a membrane, which is determined by the size, charge, and solubility of the particles.
- Temperature: Increased temperature leads to higher kinetic energy of the particles, resulting in faster diffusion.
- Molecular Size: Smaller molecules tend to diffuse faster than larger molecules due to their higher mobility.
Measuring Diffusion
Diffusion can be measured using various techniques, such as:
- Weight Measurements: The weight of a solution can be measured over time to determine the rate of diffusion. For example, if a small molecule is placed in one side of a dialysis tube and distilled water is placed on the other side, the weight of the solution in the tube can be measured to calculate the rate of diffusion.
- Concentration Measurements: The concentration of a substance on both sides of the membrane can be measured to determine the direction and rate of diffusion.
- Radioactive Tracers: Radioactive isotopes can be used to track the movement of specific particles, allowing for the measurement of diffusion rates.
Understanding Osmosis
Osmosis is a specific type of diffusion that involves the movement of water molecules across a selectively permeable membrane. This membrane only allows certain types of particles, usually small molecules like water, to pass through while blocking larger molecules. Osmosis occurs when there is a difference in solute concentration between two solutions separated by a selectively permeable membrane. Water molecules move from the solution with a lower solute concentration (hypotonic solution) to the solution with a higher solute concentration (hypertonic solution) in order to equalize the concentration of solutes on both sides of the membrane.
Factors Affecting Osmosis
The rate of osmosis is influenced by several factors:
- Solute Concentration: The greater the difference in solute concentration between the two solutions, the faster the rate of osmosis.
- Membrane Permeability: The ease with which water molecules can pass through the membrane, which is determined by the size and charge of the membrane pores.
- Temperature: Increased temperature can increase the kinetic energy of water molecules, leading to faster osmosis.
- Pressure: Applying pressure to the solution with the higher solute concentration can slow down or even reverse the direction of osmosis.
Measuring Osmosis
Osmosis can be measured using various techniques, such as:
- Tonicity Measurements: The tonicity of solutions, which refers to the concentration of solutes relative to another solution, can be used to determine the direction and rate of water movement.
- Volume Changes: The changes in the volume of solutions over time can be used to measure the rate of osmosis. For example, in Exercise B of the lab, the volume changes of the solutions inside the dialysis tubes can be used to determine the direction and rate of osmosis.
- Pressure Changes: The changes in pressure on the two sides of the membrane can be used to measure the rate of osmosis, as the movement of water molecules can create a pressure difference.
Diffusion vs. Osmosis: Key Differences
While diffusion and osmosis share the common principle of moving particles from an area of higher concentration to an area of lower concentration, they have several key differences:
- Particles Involved: Diffusion involves the movement of any type of particle, while osmosis specifically involves the movement of water molecules.
- Membrane Selectivity: Diffusion can occur through any type of membrane, including non-selective membranes, while osmosis requires a selectively permeable membrane that only allows certain particles to pass through.
- Driving Force: Diffusion is driven by the random motion of particles, while osmosis is driven by the difference in solute concentration between the two solutions.
- Equilibrium: Diffusion continues until equilibrium is reached, where the concentration of particles is equal on both sides of the membrane. Osmosis, on the other hand, continues until the solute concentration is equal on both sides of the membrane.
Practical Applications of Diffusion and Osmosis
Diffusion and osmosis have numerous practical applications in various fields, including:
- Biology and Physiology: Diffusion and osmosis play crucial roles in the transport of nutrients, gases, and waste products in living organisms, such as the exchange of oxygen and carbon dioxide in the lungs and the movement of water and solutes across cell membranes.
- Medicine: Understanding the principles of diffusion and osmosis is essential for the development of drug delivery systems, the treatment of medical conditions like edema and dehydration, and the design of dialysis machines.
- Environmental Science: Diffusion and osmosis are important processes in the movement of water and nutrients in soil, the desalination of seawater, and the purification of water.
- Industrial Applications: Diffusion and osmosis are used in various industrial processes, such as the separation of gases, the production of desalinated water, and the development of membrane-based technologies for water treatment and energy production.
Conclusion
Diffusion and osmosis are two fundamental biological processes that involve the movement of particles across a membrane. While they share the common principle of moving particles from an area of higher concentration to an area of lower concentration, they differ in the specific particles involved and the type of membranes through which they occur. Understanding the principles and factors affecting diffusion and osmosis is crucial for various fields, including biology, medicine, environmental science, and industry.
References:
– Osmosis and Diffusion
– Diffusion and Osmosis
– Osmosis Lab Example 2
– Lab 5: Diffusion and Osmosis
– Osmosis-Diffusion Background

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.