Diazomethane, which has a diazo group attached to a methylene group, is the most basic form of the diazo compounds. Now, look at its chemical applications.
Diazomethane can be used for,
- Carbene formation
- Cyclopropanation
- Methylation
- Methylation of functional group (RCOOH, Phenol, HCl, R2NH, lactums)
- Ring expansion of ketones
- Pyrazolines formation
- Wolff rearrangement reaction
- Arndt–Eistert homologation reaction
Carbenes formation
Diazomethane can produce carbenes when light energy promotes nitrogen loss from diazomethane without protonation.
- Carbenes are a class of neutral molecules with just six valence electrons. Only two hydrogen atoms are bonded with the carbon atom in it.
- Carbene, specifically “methylene carbene,” is a chemical species with very high chemical reactivity.
- A wide range of chemicals can be synthesized using methylene carbene produced from diazomethane.

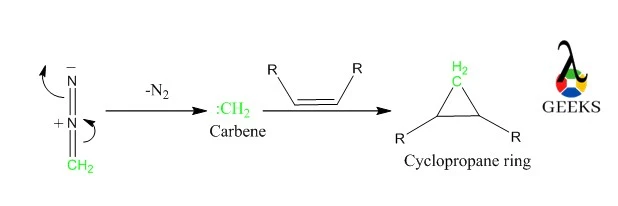
Cyclopropanation
Diazomethane forms cyclopropane ring when reacts with an alkene.
- Nitrogen accepts the pair of electrons in the N-C bond and breaks out as N2, leaving carbon with a pair of electrons and an empty orbital.
- A carbene is formed with a neutral, divalent carbon atom with one unpaired electron and an empty orbital.
- Then it reacts with an alkene to form a cyclopropane ring.
- Here is the path:
Methylation
The ability of diazomethane to substitute a methyl group for a mobile hydrogen atom, often known as methylation, underlies one of its most important applications.
Methylation of functional groups
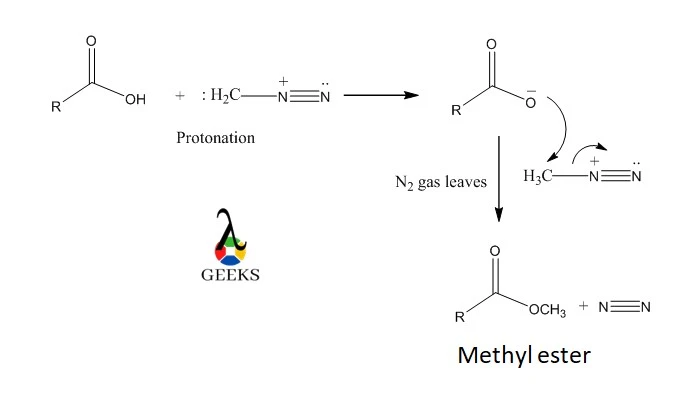
Methylation of carboxylic acid
- Diazomethane is used to O-methyl esterify carboxylic acid. The carboxylic acid first protonates diazomethane to produce CH3-N2. As N2 is one of the greatest leaving groups, this is now a fantastic alkylating agent.
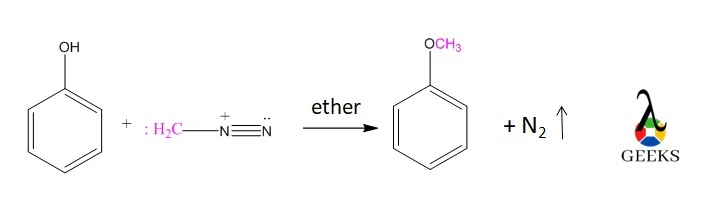
Methylation of Phenol
- Diazomethane transforms phenol to phenyl ether.
Methylation of HCl
- Diazomethane transforms hydrochloric acid into methyl chloride.

Methylation of 2° amine
- Diazomethane transforms 2° amine into 3° amine.

Methylation of lactams
- With diazomethane, lactams can be methylated in presence of catalysts (methanol, water, aluminum isopropylate, fluoboric acid).

- Otherwise, N-diazomethane is used to methylate thiolactone, heterocyclic amino compounds, and heterocyclic enols.
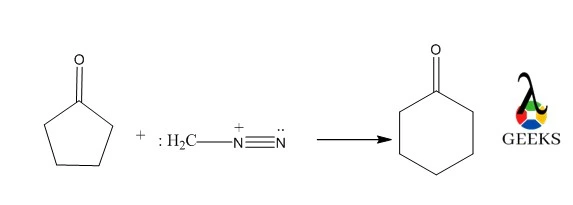
Ring expansion of ketones
When the substrate ketone is cyclic, the reaction of diazomethane with cyclopentanone is facilitated by Büchner-Curtius-Schlotterbeck, which results in one carbon ring expansion.
- The ability to produce more unstable 7- and 8-membered rings and 5- and 6-membered rings through the Büchner ring expansion reactions using diazomethane has made them desirable for synthetic purposes.
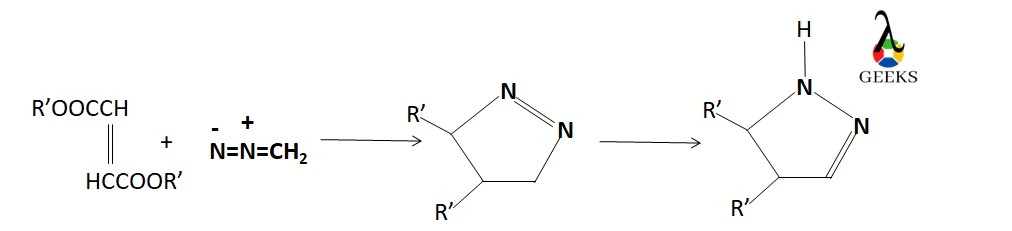
Pyrazolines formation
Diazomethane can be added to heterocycles containing “olefinic” C=C double bonds to create pyrazolines.
Wolff rearrangement
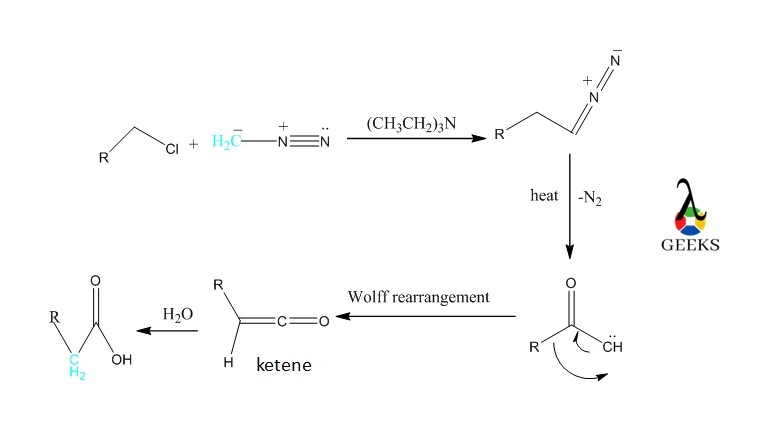
Diazomethane will combine with acid chlorides. The diazo species undergoes a dramatic transition when heated or exposed to metal like silver. Nitrogen is removed, and an arrangement is changed. One additional carbon has been added to carboxylic acid due to the addition of water. It’s known as the Wolff rearrangement.

Arndt–Eistert homologation
Reacting activated diazomethane with carboxylic acids and then Wolff-Rearranging the intermediate diazo ketones in the presence of nucleophiles such as water or amines, the Arndt-Eistert Synthesis enables the synthesis of homologated carboxylic acids or their derivatives.
Conclusion
The N-diazomethane reaction with other chemical substances has several advantageous effects, such as restricting a molecule’s flexibility to cause conformational pre-orientation. In organic chemistry, diazomethane (CH2N2) is a very important and adaptable building component.

Hey readers, I am Ishita Ghosh. I have done my Master’s in Chemistry. My area of specialization is Inorganic Chemistry. The true way to comprehend chemistry is to understand it from its grassroots level. My effort is to share every bit of knowledge in chemistry I have so that it helps you for a better grasp on this subject.