Diastereomers example or diastereomers are the types of stereoisomers that are not mirror-images of each other and have one or two chiral centers having different configurations. Whenever diastereomers example are formed then they are non-superimposable and the structures show the difference in properties in various organic reactions.
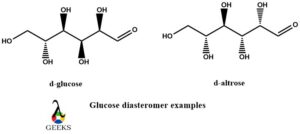
- D-glucose and D-altrose diastereomers example
- (R, R)-2-bromo-3-chlorobutane and (S,R)-2-bromo-3-chlorobutane diastereomers example
- Cis-2-butene and trans-2-butene diastereomers example
- 1-Bromo-5-ethyl cyclohexane diastereomers example
- Ribitol and Xylitol diastereomers example
- (D)-ribose, (D)-arabinose, (D)-xylose and (D)-lyxose diastereomers example
- D-Tartaric acid and meso Tartaric acid diastereomers example
- L-Tartaric acid and meso tartaric acid diastereomers example
- E-butenedioic acid (Fumaric acid) and Z-Butenedioic acid(Maleic acid) diastereomers example
- D-erythrose and D-threose diastereomers example
D-glucose and D-altrose diastereomers example
Both of these diastereomers examples belong to the carbohydrates group. In both of them, the chiral carbons are surrounded by the same type of substrates or molecules, but the difference lies in their positioning which in turn affects their configuration. The stereogenic centers are also different and the distinguished configurations are R and S. The diagrammatic representation gives more insight into it.
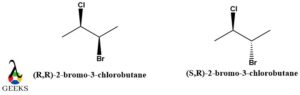
(R,R)-2-bromo-3-chlorobutane and (S,R)-2-bromo-3-chlorobutane diastereomers example
The basic structure of the above-mentioned diastereomer examples is 2-Bromo-3-chlorobutane. Many experiments are evident in the fact that 2-Bromo-3-chlorobutane is a mixture of 28% (S, R)-2-Bromo-3-chlorobutane and 18% (R, R)-2-Bromo-3-chlorobutane. Both of these diastereomer examples are non-identical and are not mirror images of each other as shown in the diagram below.
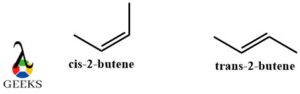
Cis-2-butene and trans-2-butene diastereomers example
Cis-2-butene and trans-2-butene are the simplest and the most common diasteromers examples. Along with that they also show the phenomenon of geometrical isomerism. Both of these structures have similar chemical properties but vary in physical properties. They have the same chemical formula. So due to differences in their orientation and arrangement, they are non-superimposable and are not mirror images of each other thereby making them the perfect diastereomer example.
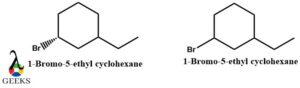
1-Bromo-5-ethyl cyclohexane diastereomers example
1-Bromo-5-ethyl cyclohexane through a single structure can be arranged in two ways based on the positioning of bromine atoms. The spatial distribution or the arrangement is different and they like any other diastereomer examples are non-mirror images.
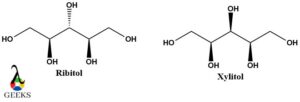
Ribitol and Xylitol diastereomers example
Ribitol and Xylitol as the name suggested belong to the hydroxyl functional group. They are pentose diastereomers and show remarkable differences in their physical properties. Both of these structures are meso compounds and are optically inactive. They also have their internal plane.
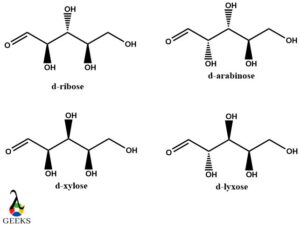
(D)-ribose, (D)-arabinose, (D)-xylose and (D)-lyxose diastereomers example
Here (D)-ribose is diastereomers of the rest of the structures. All of these are aldopentoses and consist of three chiral centers. Along with being diastereomer examples, they are also epimers. The noticeable thing in all the 4 structures drawn below is the variation in configuration at one chiral or stereocentres.
For instance (D)-ribose and (D)-arabinose differ at C-2 position
(D)-ribose and (D)-xylose differ at C-3 position
(D)-ribose and (D)-lyxose differ at both C-2 and C-3 positions.
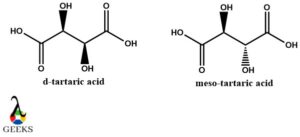
D-Tartaric acid and meso Tartaric acid diastereomers example
Tartaric acid is a very important organic compound that is found naturally in fruits and vegetables and can be artificially synthesized. Its structure is also very interesting to look at as it participates in isomerism in different forms. Meso tartaric acid is achiral and is artificially synthesized, so its properties do not show any similarity with D-Tartaric acid. Also, their central carbons are not mirror images of each hence proving it as a diastereomer example.
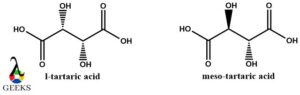
L-Tartaric acid and meso tartaric acid diastereomers example
Discussing diastereomer examples then L-tartaric acid and meso-tartaric acid are on similar lines to D-tartaric acid and meso-tartaric acid. Here they are non-superimposable and differ in the plane of symmetry. The meso tartaric acid is achiral and has a plane of symmetry.
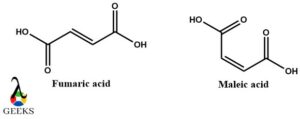
E-butenedioic acid (Fumaric acid) and Z-Butenedioic acid (Maleic acid) diastereomers example
Both fumaric acid and maleic acid are the Trans and a cis isomer of butenedioic acid respectively. They are the best diastereomeric example that has double bonds and is also geometrical isomers. The double bond has carboxylic acids on either side and they can be adjacent or opposite.
D-erythrose and D-threose diastereomers example
Both of these diastereomer examples are aldotetroses and have 2 chiral centers. In both of them, the chiral center far away from aldehyde has a hydroxyl group on the right side. The other chiral centers have a hydroxyl group on the right in erythrose and on left in threose. This makes both the diastereomer examples epimers as well.

What is a chiral center in diastereomer examples?
The chiral center is usually given importance in those organic molecules which show stereochemistry. It can be defined as an atom that is surrounded by 4 completely different or unique atoms or functional groups. In most cases, the chiral center is the carbon atoms but there are scenarios in chemistry where phosphorous, sulfur, nitrogen, and metal atoms have acted as chiral centers. Chiral centers are usually asymmetric and are non-superimposable over their mirror image.
What is the difference between diastereomer examples and enantiomer examples?
| Diasteromers | Enantiomers |
| They are not mirror images of each other | They are mirror images of each other |
| They are usually found in pairs | They are found as individual molecules |
| Their molecular shape representation is the same | They exhibit different molecular shape |
| They have similar physical properties but their ability to rotate the plane-polarized light is different. | They have different physical properties because the shape of the molecule is distinct. |
Name the technique through which various diastereomers example of a compound can be separated?
Various diastereomer examples which we have discussed before can be easily separated because of the difference in their physical properties. The most common methods are fractional distillation, recrystallization, column chromatography, HPLC (High-Performance Liquid Chromatography), thin-layer chromatography, and gas chromatography.

Hello, I am Mansi Sharma, I have completed my master’s in Chemistry. I personally believe that learning is more enthusiastic when learnt with creativity. I am a Subject Matter Expert in Chemistry.
Let’s connect through LinkedIn: https://www.linkedin.com/in/mansi-sharma22