Chloroform, also known as trichloromethane, is a dense, colorless liquid with a characteristic sweet, ethereal odor. Its density is a crucial property that has significant implications in various scientific and industrial applications. This comprehensive guide delves into the intricacies of chloroform density, providing a wealth of technical details and practical insights for physics students and enthusiasts.
Understanding Chloroform Density
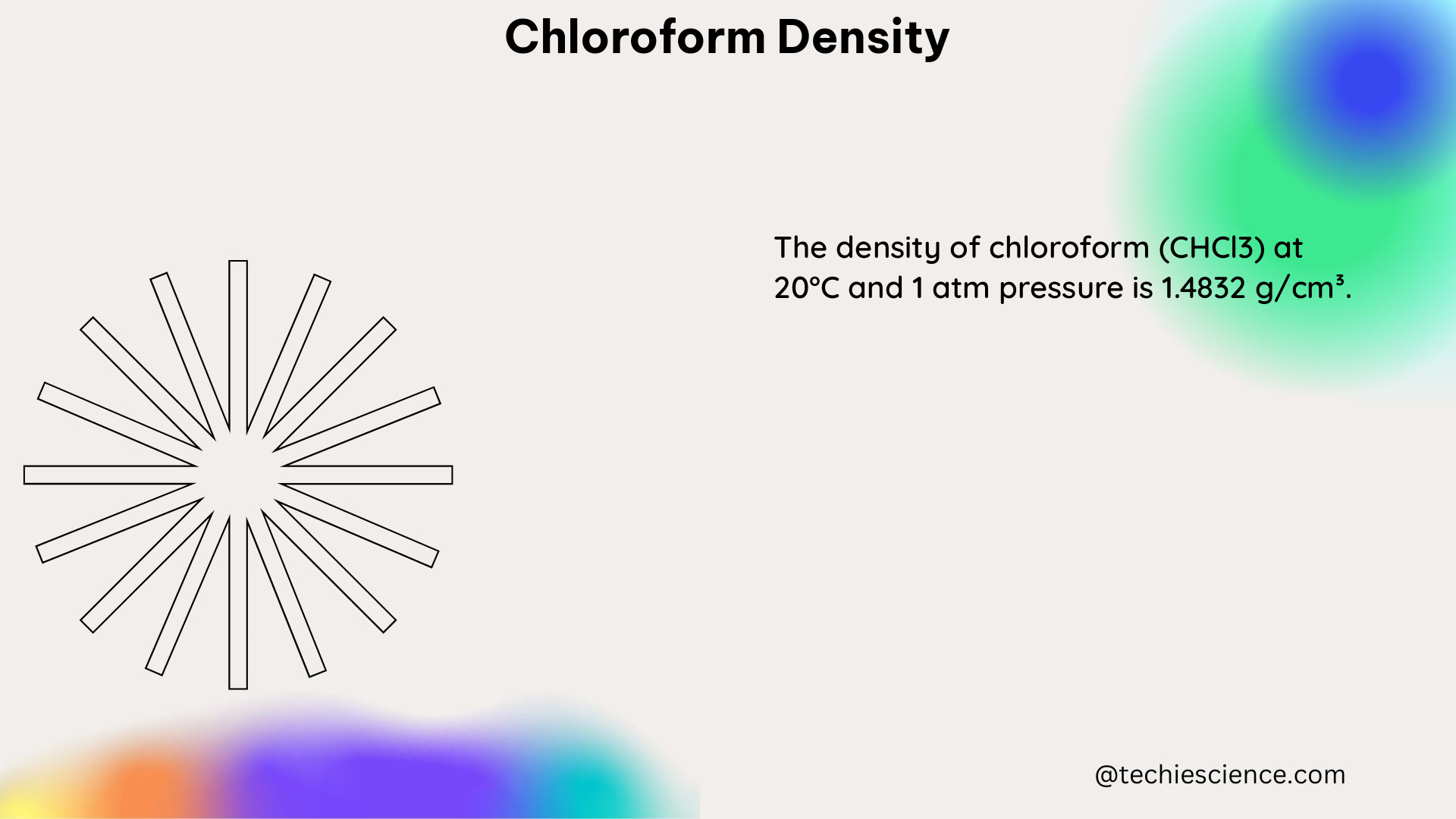
Chloroform has a density of 1.4832 g/mL at 20°C, which means that one milliliter of the liquid has a mass of approximately 1.4832 grams. This density is affected by temperature, with the value decreasing as the temperature increases. The relationship between chloroform density and temperature can be expressed using the following formula:
ρ = ρ₀ - α(T - T₀)
Where:
– ρ is the density of chloroform at the desired temperature (g/mL)
– ρ₀ is the density of chloroform at the reference temperature (1.4832 g/mL at 20°C)
– α is the coefficient of thermal expansion for chloroform (0.0011 per °C)
– T is the desired temperature (in °C)
– T₀ is the reference temperature (20°C)
Using this formula, we can calculate the density of chloroform at different temperatures:
| Temperature (°C) | Density (g/mL) |
|---|---|
| -20 | 1.564 |
| 0 | 1.527 |
| 20 | 1.4832 |
| 25 | 1.489 |
| 40 | 1.441 |
| 60 | 1.394 |
It’s important to note that the density of chloroform is significantly higher than the density of water, which is approximately 1 g/mL at room temperature. This means that chloroform will sink to the bottom of a container if mixed with water.
Factors Affecting Chloroform Density
The density of chloroform can be influenced by various factors, including:
-
Temperature: As mentioned earlier, the density of chloroform decreases as the temperature increases. This is due to the thermal expansion of the liquid, which causes the molecules to occupy a larger volume.
-
Impurities: The presence of impurities in the chloroform sample can affect its density. Impurities can alter the mass-to-volume ratio, leading to variations in the measured density.
-
Pressure: The density of chloroform is also affected by pressure. As the pressure increases, the density of the liquid will increase due to the compression of the molecules.
-
Purity: The purity of the chloroform sample can also influence its density. Highly pure chloroform will have a higher density compared to chloroform with a lower purity level.
Experimental Determination of Chloroform Density
To determine the density of chloroform experimentally, you can use various methods, such as:
-
Pycnometry: This method involves measuring the mass of a known volume of chloroform using a calibrated pycnometer. The density can then be calculated by dividing the mass by the volume.
-
Buoyancy method: In this method, a known mass of chloroform is placed in a container, and the volume of the liquid is measured. The density can then be calculated by dividing the mass by the volume.
-
Hydrometer method: A hydrometer can be used to measure the density of chloroform directly. The hydrometer is calibrated to provide the density reading when immersed in the liquid.
-
Oscillating U-tube method: This method uses an oscillating U-shaped glass tube filled with the chloroform sample. The period of oscillation is related to the density of the liquid, which can be used to calculate the density.
When conducting experiments to determine the density of chloroform, it is essential to carefully control the temperature and ensure the purity of the sample to obtain accurate and reliable results.
Chloroform Density Calculations
The density of chloroform can be used to calculate various other properties, such as the mass of a given volume of the liquid. The formula for this calculation is:
m = ρ × V
Where:
– m is the mass of the chloroform (in grams)
– ρ is the density of chloroform (in g/mL)
– V is the volume of the chloroform (in mL)
For example, if you have 185 mL of chloroform and you want to find its mass, you can use the formula:
m = 1.4832 g/mL × 185 mL = 274.09 g
Therefore, the mass of 185 mL of chloroform is approximately 274.09 grams.
Chloroform Density in Scientific Applications
The density of chloroform has various applications in the scientific and industrial fields, including:
-
Chemical Synthesis: Chloroform is used as a solvent in organic synthesis reactions, and its density is an important parameter in determining the solubility and partitioning of reactants and products.
-
Forensic Science: Chloroform is sometimes used in forensic investigations, and its density can be used to identify the presence and quantity of the substance in samples.
-
Pharmaceutical Industry: Chloroform is used in the production of certain pharmaceutical drugs, and its density is a critical parameter in the formulation and quality control processes.
-
Environmental Studies: The density of chloroform is relevant in environmental studies, as it can affect the transport and fate of the substance in soil and groundwater systems.
-
Analytical Chemistry: Chloroform is commonly used as a solvent in analytical techniques, such as liquid-liquid extraction, and its density is an important factor in the efficiency and accuracy of these methods.
Conclusion
In this comprehensive guide, we have explored the intricacies of chloroform density, covering its measurement, factors affecting it, and its applications in various scientific and industrial fields. By understanding the technical details and practical implications of chloroform density, physics students and enthusiasts can gain a deeper appreciation for this important property and its role in the world of science and technology.
References:
- Lide, D. R. (2005). CRC Handbook of Chemistry and Physics (86th ed.). CRC Press.
- Weast, R. C. (1984). CRC Handbook of Chemistry and Physics (64th ed.). CRC Press.
- Haynes, W. M. (2014). CRC Handbook of Chemistry and Physics (95th ed.). CRC Press.
- Chloroform. (n.d.). In Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Chloroform
- Chloroform Density. (n.d.). In PubChem. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Chloroform#section=Density

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.