Cl or Chlorine is one of the most electronegative elements in the periodic table and belongs to the halogen family. Let us explore the electronegativity and ionization of Cl.
Chlorine is the 2nd member of the group 17th halogen family after fluorine. Down the group electronegativity decreases due to an increase in atomic size and a lowering of the effective nuclear charge, so Cl is less electronegative than F. The ionization of Cl required higher energy as it is strong electronegative.
At room temperature, it can exist as a gaseous phase so it has higher vapor pressure. In this article, we should learn the electronegativity property and ionization energy and comparison of electronegativity between Chlorine and other elements in detail with proper explanation.
1. Why chlorine has the highest electron affinity
Cl has the highest electron affinity among the other elements in the periodic table due to the following reasons –
- Cl has higher electronegativity so it can pull sigma electron density from another electron source.
- Cl has a vacant d- orbital so it can accept the excess electron density in there
- Due to the larger size of Cl, it can easily accumulate extra electrons and there is no lone-pairs repulsion, unlike F.
2. Chlorine and sodium electronegativity
The electronegativity of Cl is 3.16 which is one of the most electronegative atoms in the periodic table now we compare the electronegativity between Cl and Na metal,
| Electronegativity of Chlorine | Electronegativity of Sodium | Reasons |
| 3.16 | 0.93 | Na is an alkali metal so it has less electronegative character rather it shows electropositive nature and easily loose electrons and the difference between the electronegativity of Na and F is almost 2.23. |
3. Chlorine and potassium electronegativity
The electronegativity difference between the halogen Cl atom and alkali metal potassium is discussed in the following table,
| Electronegativity of Chlorine | Electronegativity of Potassium | Reasons |
| 3.16 | 0.86 | K lies down the Na in group IA so it has less electronegativity than Na whereas Cl is a group VIIA element so it has higher electronegativity and the difference between them is 2.3. |
4. Chlorine and carbon electronegativity
Cl is more electronegative than carbon because Cl belong to the most electronegative group halogen family where C lies in group 14 which is far away from it and the comparison between them is –
| Electronegativity of Chlorine | Electronegativity of Carbon | Reasons |
| 3.16 | 2.55 | C lies in group 14th which is closer to groups 15 and 16 but far away from group 17 so it has a lower electronegativity than the halogen element like Cl. |
5. Chlorine and nitrogen electronegativity
Cl is a group VIIA element whereas nitrogen is Group 15th element so it has a lower electronegativity than Cl which can be described below –
| Electronegativity of Chlorine | Electronegativity of Carbon | Reasons |
| 3.16 | 3.04 | N is group15th pnictogen element so it has moderate electronegativity but across the period from left to right electronegativity increases and Cl presents a far-right site to N so it has more electronegativity and the difference between them is 0.12 only. |
6. Chlorine and oxygen electronegativity
The electronegativity of O is more than Cl although Cl belongs to halogen and o belongs to chalcogen and the reason is –
| Electronegativity of Chlorine | Electronegativity of Oxygen | Reasons |
| 3.16 | 3.04 | Cl is present in group 17th of the halogen family but it has a lower electronegativity than O because Cl is present down the group than O and it has lowers effective nuclear charge so having lower electronegativity, whereas O is present in the top position of group 16th. |
7. Chlorine and bromine electronegativity
Chlorine and bromine both belong to the same group but Cl has higher electronegativity and the reason is described in the following table,
| Electronegativity of Chlorine | Electronegativity of Bromine | Reasons |
| 3.16 | 2.96 | Cl and Br both lie in the halogen group and bromine is present next to Cl so it has a higher atomic size and lowers effective nuclear charge. So, Br has a lower sigma electron density as the increase of principle quantum number than Cl. |
8. Ca and Cl electronegativity
Ca has a lower electronegativity than Cl as calcium is an alkaline earth metal whereas Cl is non-metal and we compare the electronegativity between them in the following table –
| Electronegativity of Chlorine | Electronegativity of Calcium | Reasons |
| 3.16 | 1 | Calcium is alkaline earth metal that is more electropositive in nature so it has a lower electronegativity than Cl whereas Cl is a halogen non-metal that has higher sigma electron density and electron affinity so it has a higher electronegativity than Ca. |
9. Si and Cl electronegativity
Silicon has a lower electronegativity than Cl as it is present in the group 14th and the difference in electronegativity is discussed below –
| Electronegativity of Chlorine | Electronegativity of Silicon | Reasons |
| 3.16 | 1.9 | Silicon has a lower electronegativity than C as it is placed lower position than C. Cl has a higher electronegativity than Si because it is placed on more right side of Si in the group 17th so it has more sigma electron density than Si and has a higher electronegativity. |
10. Chlorine ionization energy
The ionization energy of Cl is higher due to the higher electronegativity of the element and it attracts the outer electrons very tightly. The first three ionization energy are discussed below –
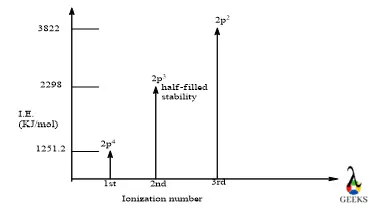
- 1st I.E. – the first I.E. of Cl is 1251.2 KJ/mol which occurs from 2p orbital.
- 2nd I.E. – the second I.E. of Cl is 2298 KJ/mol which also occurs from 2p orbital and it gains stability after 2nd ionization so it is comparatively low.
- 3rd I.E. – the third ionization energy for Cl is 3822 KJ/mol and upon third ionization, it lost its half-filled stability and required more energy.
11. Chlorine ionization energy graph
The electronic configuration of Cl is [Ne]3s23p5 so the first, second, and third ionization occurs from its 2p orbital respectively, and corresponding energy is presented in the graphical form below –
12. Chlorine and aluminum ionization energy
Al belongs to group 13 whereas Cl is present in group 17th so they have different ionization energy which is discussed below –
| Ionization | Chlorine | Aluminum | Reasons |
| 1st | 1251.2 KJ/mol | 577.5 KJ/mol | Al is less electronegative than Cl and upon the first ionization, it gets stabilized which is removed from its 2p orbital. |
| 2nd | 2298 KJ/mol | 1816.7 KJ/mol | Upon 2nd ionization, Cl gets half-filled stability whereas for Al it loses its stability and the electron is removed from 2s orbital which is closer to the nucleus so it required higher energy. |
| 3rd | 3822 KJ/mol | 2744.4 KJ/mol | On the third ionization, Cl loses its stability and for Al, it is removed from the +2 excited state and the 2s orbital. |
13. Chlorine and oxygen ionization energy
O and Cl both lie in a different group, the former is chalcogen and the latter is halogen so their ionization will be different which is discussed below in table –
| Ionization | Chlorine | Oxygen | Reasons |
| 1st | 1251.2 KJ/mol | 1313.9 KJ/mol | O is more electronegative than Cl so its first ionization energy is higher than Cl. |
| 2nd | 2298 KJ/mol | 3388.3 KJ/mol | Upon 2nd ionization O lost its half-filled stabilization and also due to higher electronegativity, it required much higher energy for removal of 2nd electron. |
| 3rd | 3822 KJ/mol | 5300.5 KJ/mol | On the third ionization for O, the electron is removed from the +2 excited state, and O has higher electronegativity so it required much higher energy than Cl. |
14. Chlorine and sulfur ionization energy
In the following table, we compare the ionization energy between Cl and S –
| Ionization | Chlorine | Sulfur | Reasons |
| 1st | 1251.2 KJ/mol | 999.6 KJ/mol | S is less electronegative than Cl and on first ionization, it gets stabilized so the I.E. of S is less than Cl. |
| 2nd | 2298 KJ/mol | 2252 KJ/mol | Upon 2nd ionization, S lost its half-filled stabilization so the ionization energy increases but is less than Cl. |
| 3rd | 3822 KJ/mol | 3357 KJ/mol | On the third ionization S loose three electrons from its valence shell and it occurs from a +2 excited state so required higher energy. |
15. Chlorine and fluorine ionization energy
Cl and F belong to the same group but they have different ionization values based on their electronegativity which is described below –
| Ionization | Chlorine | Fluorine | Reasons |
| 1st | 1251.2 KJ/mol | 1681 KJ/mol | F is the most electronegative atom so it has a higher attraction towards valence electrons so it required much more energy for ionization. |
| 2nd | 2298 KJ/mol | 3374.2 KJ/mol | On 2nd ionization, F gets stabilized by a half-filled configuration but the electron is removed from the +1 excited state so higher energy will be required. |
| 3rd | 3822 KJ/mol | 6050.4 KJ/mol | On the third ionization, f lost its stability and the electron was removed from the +2 excited state so for these reasons it required much more energy which is almost double than previous one. |
Conclusion
Cl is one of the most electronegative atoms present in the halogen family. Due to its higher electronegativity, it can attract a sigma bond towards itself. It has a higher electron affinity and for this reason, it can easily form ionic bonds.
Read more about Aluminum Electronegativity & Ionization Energy, Barium Electronegativity & Ionization Energy, Cesium Ionization Energy & Electronegativity and Antimony Electronegativity & Ionization Energy.

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.