Calcium hydroxide, also known as slaked lime or hydrated lime, is a sparingly soluble salt that plays a crucial role in various chemical and industrial processes. Understanding the solubility of calcium hydroxide in water is essential for applications ranging from water treatment to construction materials. This comprehensive guide delves into the intricacies of calcium hydroxide solubility, providing a wealth of technical details and practical insights for science students and professionals.
The Solubility Product Constant (Ksp) of Calcium Hydroxide
The solubility of calcium hydroxide in water is governed by the solubility product constant (Ksp), which is a measure of the solubility of a sparingly soluble salt. The Ksp of calcium hydroxide is defined as the product of the concentrations of the calcium ions (Ca2+) and hydroxide ions (OH-) raised to their respective stoichiometric coefficients in the dissolution reaction:
Ksp = [Ca2+][OH-]2
The value of the Ksp for calcium hydroxide at 25°C is 6.5 × 10-6. This Ksp value can be used to predict the solubility of calcium hydroxide in water under various conditions, such as changes in temperature and concentration.
Determining the Ksp of Calcium Hydroxide
The Ksp of calcium hydroxide is typically determined through laboratory experiments. One common method involves the following steps:
- Prepare a saturated solution of calcium hydroxide in water.
- Titrate the saturated solution with a standard solution of hydrochloric acid (HCl).
- Monitor the pH of the solution during the titration using a pH meter to determine the endpoint.
- Calculate the concentration of OH- ions in the saturated solution using the stoichiometry of the reaction between Ca(OH)2 and HCl.
- Determine the Ksp value using the expression Ksp = [Ca2+][OH-]2.
By following this procedure, researchers can accurately measure the Ksp of calcium hydroxide and use the obtained value to study its solubility behavior.
Factors Affecting the Solubility of Calcium Hydroxide
The solubility of calcium hydroxide in water can be influenced by several factors, including temperature, concentration, and the presence of other ions in the solution.
Temperature
The solubility of calcium hydroxide in water is inversely proportional to temperature. As the temperature increases, the solubility of calcium hydroxide decreases, and the Ksp value also decreases. This relationship can be expressed using the van ‘t Hoff equation:
ln(Ksp2/Ksp1) = -ΔH°/R × (1/T2 – 1/T1)
where ΔH° is the standard enthalpy change of the dissolution reaction, R is the universal gas constant, and T1 and T2 are the absolute temperatures.
Concentration
The concentration of calcium hydroxide in a solution also affects its solubility. As the concentration of Ca(OH)2 increases, the concentrations of Ca2+ and OH- ions also increase, leading to a higher Ksp value. Conversely, if the concentration of Ca(OH)2 decreases, the Ksp value will also decrease.
Presence of Other Ions
The presence of other ions in the solution can influence the solubility of calcium hydroxide. For example, the addition of a common ion, such as calcium or hydroxide ions, can decrease the solubility of calcium hydroxide due to the common ion effect. Conversely, the presence of complexing agents or chelating agents that can bind to calcium ions can increase the solubility of calcium hydroxide.
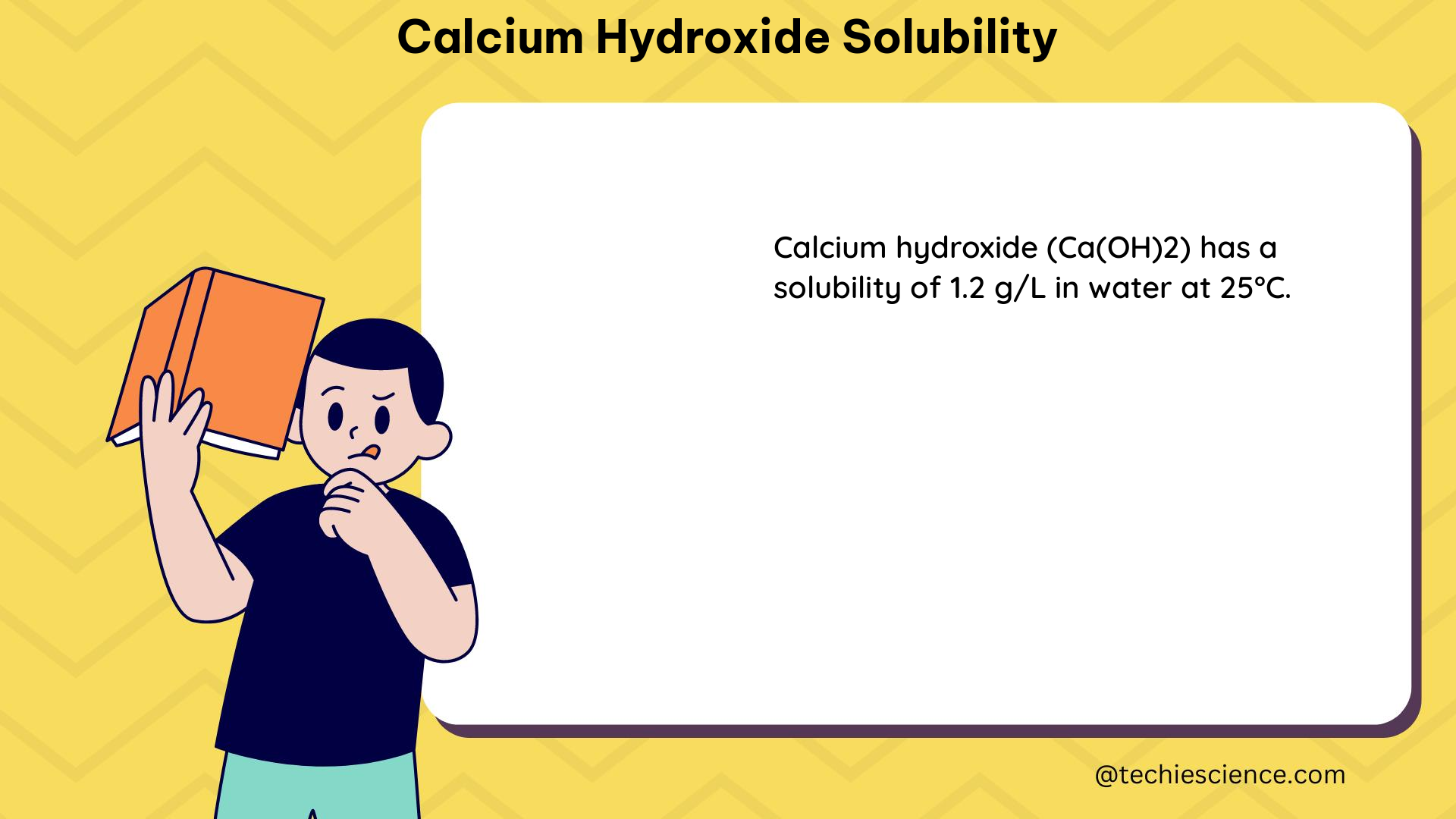
Solubility of Calcium Hydroxide in Water
In addition to the Ksp value, the solubility of calcium hydroxide in water can also be expressed in terms of the mass of the salt that dissolves in a given volume of water at a specific temperature. At 25°C, the solubility of calcium hydroxide in water is approximately 1.7 g/L.
This solubility value can be used to predict the amount of calcium hydroxide that will dissolve in a given volume of water at a specific temperature. For example, if you have 100 mL of water at 25°C, the maximum amount of calcium hydroxide that can dissolve in this volume is:
1.7 g/L × (0.1 L) = 0.17 g
Applications of Calcium Hydroxide Solubility
The understanding of calcium hydroxide solubility has numerous applications in various fields, including:
-
Water Treatment: Calcium hydroxide is used in water treatment processes, such as softening and pH adjustment, due to its ability to react with dissolved carbon dioxide and remove hardness from water.
-
Construction Materials: Calcium hydroxide is a key component in the production of cement and concrete, where its solubility and reactivity play a crucial role in the setting and hardening of these materials.
-
Agriculture: Calcium hydroxide is used as a soil amendment to adjust the pH of acidic soils, improving nutrient availability and plant growth.
-
Chemical Processes: The solubility of calcium hydroxide is important in various chemical processes, such as the production of calcium carbonate, the neutralization of acidic waste streams, and the synthesis of other calcium-based compounds.
-
Pharmaceutical and Cosmetic Industries: Calcium hydroxide is used in the production of some pharmaceutical and cosmetic products, where its solubility and pH-adjusting properties are essential.
Understanding the solubility of calcium hydroxide is crucial for optimizing these and other applications, ensuring efficient and effective use of this versatile chemical compound.
Conclusion
Calcium hydroxide is a sparingly soluble salt with a well-defined solubility product constant (Ksp) that can be used to predict its solubility in water under various conditions. By delving into the technical details of calcium hydroxide solubility, including the factors that influence it and the methods used to determine the Ksp, this comprehensive guide provides a valuable resource for science students and professionals working in fields where the understanding of calcium hydroxide solubility is crucial.
References
- Determination of the Solubility Product Constant (Ksp) of Calcium Hydroxide. (n.d.). Retrieved from https://www.coursehero.com/file/230158547/Lab-Report-Determination-of-the-Solubility-Product-Constant-Ksp-of-Calcium-Hydroxidepdf/
- Experiment 4: Determining the Solubility Product Constant (Ksp) of Calcium Hydroxide. (n.d.). Retrieved from https://www.studocu.com/en-us/document/howard-community-college/general-inorganic-chemistry/exp-4-determining-the-solubility-product-constant-ksp-of-calcium-hydroxide/8401646
- Experiment 1: Finding the Solubility of Calcium Hydroxide by Three Different Methods. (n.d.). Retrieved from https://quizlet.com/au/778416862/experiment-1-finding-the-solubility-of-calcium-hydroxide-by-three-different-methods-flash-cards/

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.