Here we will see how to balance C4h6o4 + Koh chemical equation and the type of reaction, their characteristics and few us. In order to balance the equation or what products are formed, it is necessary to know what the reagents are.
C4H6O4 is the chemical formula for Succinic acid, which is an important intermediate organic molecule/metabolite in TCA cycle. It is a water soluble colorless alpha, omega- dicarboxylic acid. Its IUPAC nomenclature is Butane-1,4-dioic acid with molecular weight of 118.09 g/mol and density 1.56 g/cm3 .
It is widely used in skincare industry, medicinal industry, manufacture of perfumes, lacquers etc. It is found naturally in foods as one of the most important acids after citric acid e.g. , in red meats, radish, beetroots, broccoli etc. Succinic acid the conjugate acid of succinate, which can be produced by hydrogenation reaction of maleic anhydride, fumaric acid or malic acid.
KOH, the chemical formula for potassium hydroxide, is used widely as aqueous as well as alkaline solution for production of organic molecules containing hydroxyl group –OH as the functional groups like ethanol, propanol or phenols.
It is known by the common name Caustic potash with molecular weight 56.2 g/mol and density of 2.12 g/cm3 . It is highly hygroscopic meaning it absorbs moisture quickly and is soluble in water. Direct contact with KOH causes extreme skin and eyes irritations and in severe cases causes burning of the skin.
What is C4h6o4 + koh ?
C4h6o4 + koh is the chemical reaction between succinic acid and potassium hydroxide. It is a neutralization reaction where an acid and a base reacts to form salt and water. In other words, it is an acid-base reaction. Succinic acid is a diprotic acid which requires two moles of base to form a salt.
Here, the base is KOH, it replaces two protons from –COOH group giving –COO–K+ . The neutralization point or the end point of the completion of reaction can be recognized by using phenolphthalein as an indicator.
Generally, an acid-base titration is carried out for C4h6o4 + koh .


What is the product of C4h6o4 + koh ?
Here, the product formed is Potassium salt of Butane-1,4-dioic acid, with simultaneous production of water molecule, an indicator of neutralization reaction. Each carboxylic group –COOH can liberate its proton and be replaced by K+ cations.
The interionic attraction are sufficient enough to keep the charges together. Also, the formation of salt can be identified by dissolving in water as it would be completely soluble in it.
C4h6o4 + koh undergoes an acid-base reaction to produce salts of carboxylic acid. Here, succinic acid is the acid and potassium hydroxide is the base. Every reaction first tends to undergo acid-base reaction if possible and by nature.
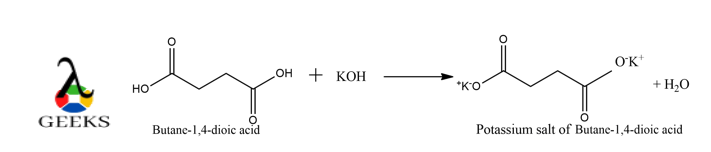
How to balance C4h6o4 + koh ?
The reaction is shown below :
xC4H6O4 + yKOH ———- > zC4H4O42-2K+ + vH2O
The simplest way to balance a chemical equation is by first writing down the coefficient i.e. , the number that is written before the reactant as “X” , “Y” and so on , which denotes the number of moles that is used to carry out the reaction to completion.
The subscript in chemical formula represents the number of each constituent element that participates to form a molecule. An element containing subscript ‘1’ means it is present as an atom.
Step 1 : Count the number of atoms of each constituent element in both sides of the reaction and form a table.
- C = 4 , H = 7 , O = 5 , K = 1 on the left side of the equation.
- C = 4 , H = 6 , O = 5 , K = 2 on the right side of the equation.
Step 2 : Balance K followed by C :
There is 1 K atom on left side and 2 K atoms on the right side, therefore, multiplying KOH by the coefficient 2 on the left side.
C atoms are already balanced on both the sides of the equation.
Step 3 : Balance H followed by O atoms :
There are total 8 H atoms on the left side and 6 on the right side, so multiplying H2O by coefficient 2 balances the H atoms. In doing so, the O atoms get simultaneously balanced.
Therefore, the complete balanced chemical equation of the acid-base reaction of succinic acid with caustic potash is shown below :
C4H6O4 + 2KOH ———- > C4H4O42-2K+ + 2H2O
What type of reaction is C4h6o4 + koh ?
C4H6O4 + KOH is a neutralization reaction, a type of acid-base titration, that produces potassium salts of succinic acid on reaching neutralization point which can be identified by using an indicator of the pH range of the reaction.
It is one of the most important reactions for mankind as it briefs about how succinic acid is maintained and produced in our biological TCA cycle where the intermediate, succinate is the most important and studied metabolite.
This helps in further production of ATP and thereby, helping in energy production. Also, it is a diprotic acid meaning two moles of base will always be required to completely neutralize the acid.
Conclusion :
Succinic acid is a diprotic acid and the above discussion helps in understanding how reaction proceeds and method to obtain a balanced equation.

Hello…. I am Nandita Biswas. I have completed my master’s in Chemistry with a specialization in organic and physical chemistry. Also, I have done two projects in chemistry- One dealing with colorimetric estimation and determination of ions in solutions. Others in Solvatochromism study fluorophores and their uses in the field of chemistry alongside their stacking properties on emission. I am working as a Research Associate Trainee in Medicinal Department.
Let’s connect through LinkedIn-https://www.linkedin.com/in/nandita-biswas-244b4b179