Butane, a versatile hydrocarbon fuel, has a density that varies depending on temperature, pressure, and phase (liquid or gas). Understanding the density of butane is crucial for its various applications, such as fuel for lighters, cooking stoves, and refrigerators. This comprehensive guide delves into the technical details and provides a hands-on resource for physics students and enthusiasts.
Understanding Butane Density
Butane, with the chemical formula C₄H₁₀, is a colorless, flammable gas that is denser than air. Its density is an essential physical property that determines its behavior and performance in various applications.
Liquid Butane Density
At atmospheric pressure and a temperature of 25°C (77°F), the density of liquid butane is approximately 581.5 kg/m³ (36.32 lb/ft³ or 0.03632 lb/in³). This density remains relatively constant at the gas-liquid equilibrium pressure, up to a pressure of 100 bara (1,450 psia).
The density of liquid butane can be calculated using the following formula:
ρ = P / (R * T)
Where:
– ρ is the density of the liquid (kg/m³)
– P is the pressure (Pa)
– R is the specific gas constant for butane (296.8 J/(kg·K) or 0.08205 lb·ft/(lb·mol·°R))
– T is the absolute temperature (K)
This formula is based on the ideal gas law, which assumes that the gas behaves as an ideal gas. However, at high pressures and low temperatures, the real gas behavior becomes significant, and the Peng-Robinson equation of state can be used to calculate the density more accurately.
Gaseous Butane Density
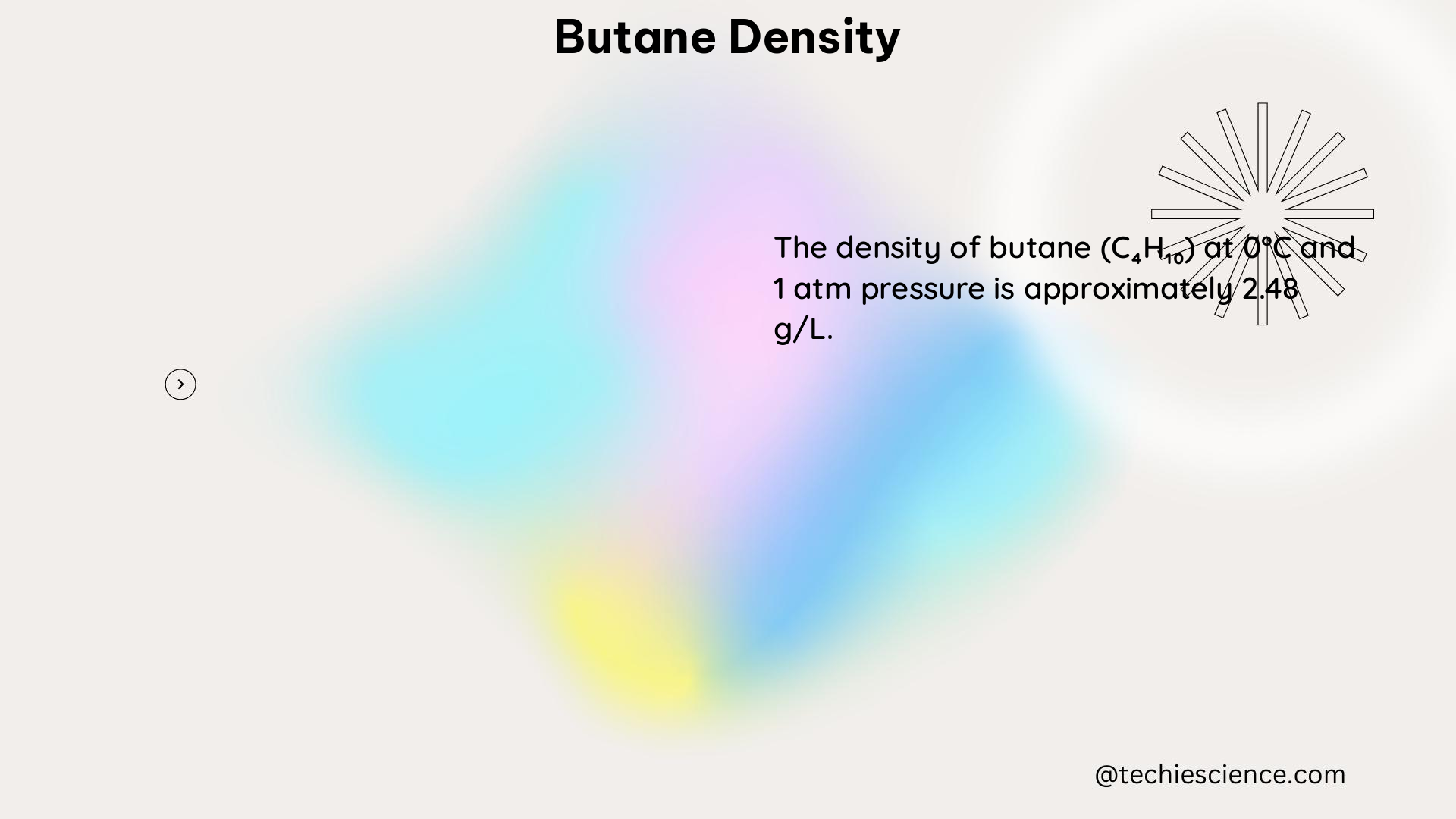
The density of gaseous butane at atmospheric pressure and 25°C (77°F) is approximately 2.48 kg/m³ (0.155 lb/ft³ or 0.000155 lb/in³). This density can also be calculated using the ideal gas law:
ρ = P / (R * T)
Where:
– ρ is the density of the gas (kg/m³)
– P is the pressure (Pa)
– R is the specific gas constant for butane (296.8 J/(kg·K) or 0.08205 lb·ft/(lb·mol·°R))
– T is the absolute temperature (K)
It’s important to note that the ideal gas law becomes less accurate at high pressures and low temperatures, where the real gas behavior becomes more significant. In such cases, the Peng-Robinson equation of state can provide a more accurate calculation of the butane density.
Factors Affecting Butane Density
The density of butane is influenced by several factors, including temperature, pressure, and the presence of impurities.
Temperature Dependence
The density of both liquid and gaseous butane is inversely proportional to temperature. As the temperature increases, the density decreases, and vice versa. This relationship can be expressed using the following equation:
ρ = ρ₀ * (T₀ / T)
Where:
– ρ is the density at the desired temperature (kg/m³)
– ρ₀ is the density at the reference temperature (kg/m³)
– T is the desired temperature (K)
– T₀ is the reference temperature (K)
For example, the density of liquid butane at 0°C (32°F) is approximately 601.8 kg/m³, while at 50°C (122°F), it is around 560.2 kg/m³.
Pressure Dependence
The density of liquid butane is relatively insensitive to pressure changes, as long as the pressure remains at the gas-liquid equilibrium. However, the density of gaseous butane is directly proportional to pressure, as described by the ideal gas law:
ρ = P / (R * T)
Where:
– ρ is the density of the gas (kg/m³)
– P is the pressure (Pa)
– R is the specific gas constant for butane (296.8 J/(kg·K) or 0.08205 lb·ft/(lb·mol·°R))
– T is the absolute temperature (K)
As the pressure increases, the density of gaseous butane also increases, and vice versa.
Impurity Effects
The presence of impurities, such as other hydrocarbons or water, can affect the density of butane. The density of butane-rich mixtures can be calculated using the volume averaging method or the Hayden-O’Connell equation, which takes into account the composition of the mixture.
Practical Applications and Numerical Examples
Butane density is crucial in various applications, including:
-
Fuel for lighters and cooking stoves: The density of butane determines the amount of fuel that can be stored in a given volume, affecting the performance and runtime of these devices.
-
Refrigeration systems: Butane is used as a refrigerant in some systems, and its density affects the system’s efficiency and capacity.
-
Chemical processing: Butane is used as a feedstock in the petrochemical industry, and its density is important for process design and optimization.
Let’s explore some numerical examples to illustrate the practical applications of butane density:
Example 1: Calculating Liquid Butane Density
Given:
– Temperature: 25°C (298 K)
– Pressure: 1 atm (101.325 kPa)
Using the ideal gas law:
ρ = P / (R * T)
ρ = 101.325 kPa / (296.8 J/(kg·K) * 298 K)
ρ = 581.5 kg/m³
This matches the reference value provided earlier, confirming the density of liquid butane at the given conditions.
Example 2: Calculating Gaseous Butane Density
Given:
– Temperature: 25°C (298 K)
– Pressure: 1 atm (101.325 kPa)
Using the ideal gas law:
ρ = P / (R * T)
ρ = 101.325 kPa / (296.8 J/(kg·K) * 298 K)
ρ = 2.48 kg/m³
This result matches the reference value for the density of gaseous butane at the given conditions.
Example 3: Density Variation with Temperature
Suppose we want to calculate the density of liquid butane at 0°C (273 K) and 50°C (323 K).
Using the temperature dependence equation:
ρ = ρ₀ * (T₀ / T)
At 0°C (273 K):
ρ = 581.5 kg/m³ * (298 K / 273 K)
ρ = 601.8 kg/m³
At 50°C (323 K):
ρ = 581.5 kg/m³ * (298 K / 323 K)
ρ = 560.2 kg/m³
These results demonstrate the inverse relationship between temperature and the density of liquid butane.
Conclusion
Butane density is a crucial physical property that plays a significant role in various applications, from fuel for lighters and cooking stoves to refrigeration systems and chemical processing. This comprehensive guide has provided a detailed exploration of the factors affecting butane density, including temperature, pressure, and the presence of impurities. By understanding the technical details and practical applications of butane density, physics students and enthusiasts can better navigate the complexities of this important hydrocarbon fuel.
References
- Density Measurements of (0.99 Methane + 0.01 Butane) and (0.98 Methane + 0.02 Isopentane) over the Temperature Range from 100 to 160 K at Pressures up to 108 MPa
- Quantitative evaluation of n-butane concentration on knock severity of a natural gas heavy-duty SI engine
- Measuring the mass, volume, and density of microgram-sized objects in fluid
- Density of Butane Gas Lab – YouTube
- Butane – Density and Specific Weight vs. Temperature and Pressure – Engineering ToolBox

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.