The boiling point of triethylamine, a widely used organic compound, is a crucial parameter in various chemical and industrial processes. This comprehensive guide delves into the intricacies of the boiling point of triethylamine, providing a wealth of technical details and practical insights for science students and professionals alike.
Understanding the Boiling Point of Triethylamine
Triethylamine, with the chemical formula (CH3CH2)3N, is a colorless, flammable liquid with a characteristic amine-like odor. The boiling point of this compound, which is the temperature at which the vapor pressure of the liquid equals the surrounding atmospheric pressure, is a fundamental property that has significant implications in various applications.
Factors Influencing the Boiling Point
The boiling point of triethylamine is influenced by several factors, including:
- Molecular Structure: The presence of three ethyl groups (CH3CH2-) attached to the nitrogen atom in the triethylamine molecule contributes to its relatively high boiling point compared to other amines.
- Intermolecular Forces: The strength of the intermolecular forces, such as van der Waals interactions and hydrogen bonding, plays a crucial role in determining the boiling point. In the case of triethylamine, the relatively weak intermolecular forces result in a lower boiling point compared to compounds with stronger intermolecular interactions.
- Atmospheric Pressure: The boiling point of triethylamine, like any other substance, is directly influenced by the surrounding atmospheric pressure. The standard boiling point is typically measured at an atmospheric pressure of 101.3 kPa (1 atm).
Theoretical Considerations
The boiling point of triethylamine can be calculated using the Clausius-Clapeyron equation, which relates the vapor pressure of a substance to its temperature:
ln(P) = (-ΔHvap/R)(1/T) + C
Where:
– P is the vapor pressure of the substance (in Pa)
– ΔHvap is the enthalpy of vaporization (in J/mol)
– R is the universal gas constant (8.314 J/mol·K)
– T is the absolute temperature (in K)
– C is a constant
By rearranging the equation and substituting the appropriate values for triethylamine, the boiling point can be determined.
Experimental Determination of the Boiling Point
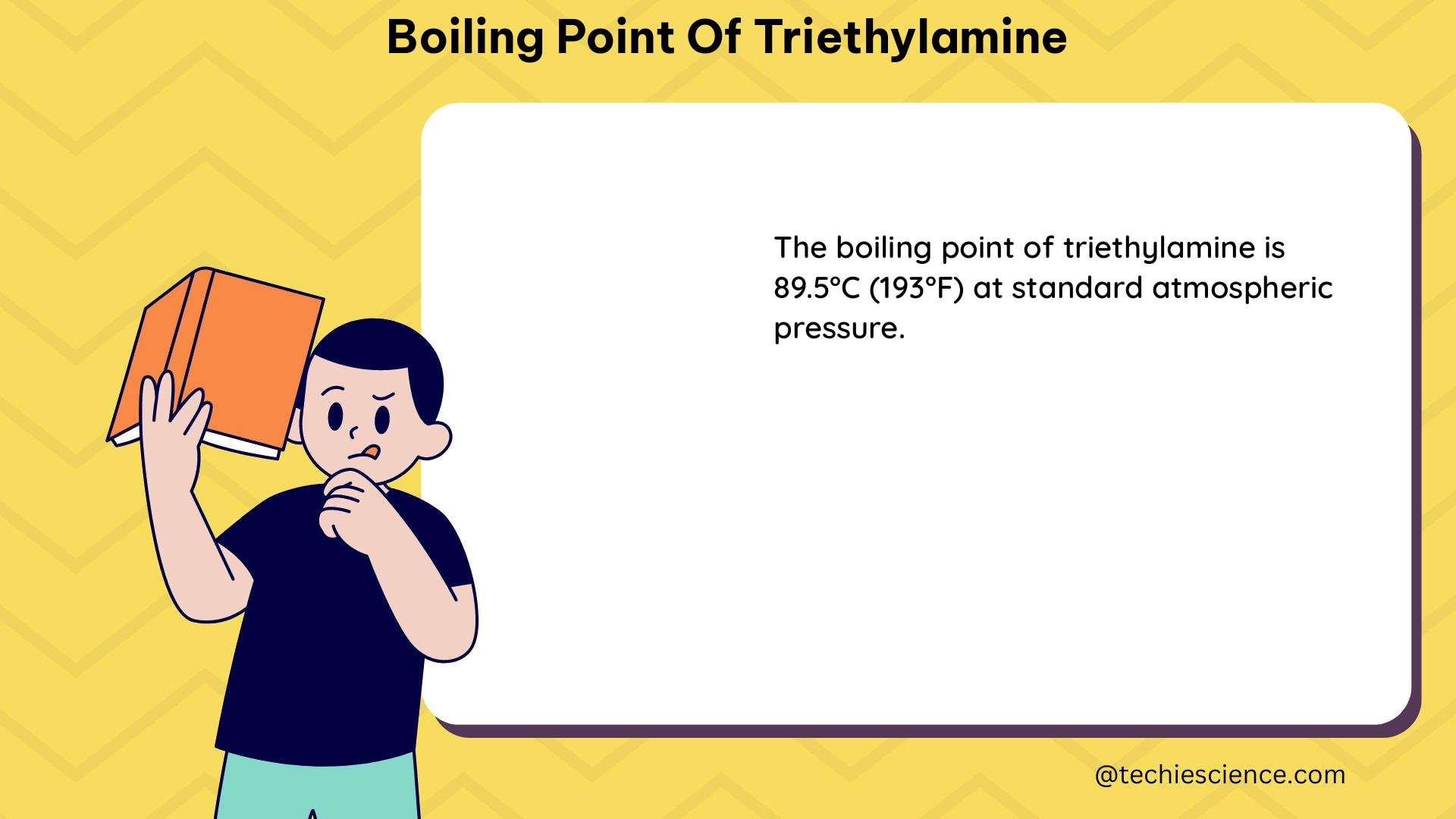
The boiling point of triethylamine has been extensively studied and reported in the literature. The most commonly cited value is 88.6 °C (361.9 K) or 191.8 °F, which is the normal boiling point of the compound.
Vapor Pressure Measurements
The boiling point of triethylamine is typically determined by measuring its vapor pressure as a function of temperature. This can be done using various experimental techniques, such as:
- Static Vapor Pressure Measurements: In this method, the vapor pressure of triethylamine is measured directly using a manometer or other pressure-measuring device.
- Dynamic Vapor Pressure Measurements: Here, the vapor pressure is determined by measuring the temperature at which the liquid boils under a controlled pressure.
The observed vapor pressure data is then used to extrapolate the boiling point at the standard atmospheric pressure of 101.3 kPa (1 atm).
Uncertainty and Consistency
The boiling point of triethylamine has an uncertainty of approximately 0.5 K, as reported in the literature. This value is consistent with the boiling point range of 88.6-88.8 °C found in other reliable sources.
Applications and Implications
The boiling point of triethylamine has significant implications in various chemical and industrial applications, including:
- Organic Synthesis: Triethylamine is a widely used base and catalyst in organic synthesis reactions, and its boiling point is crucial in determining the appropriate reaction conditions and separation techniques.
- Solvent Applications: Triethylamine is employed as a solvent in various chemical processes, and its boiling point is a key parameter in solvent selection and recovery.
- Pharmaceutical and Agrochemical Industries: Triethylamine is used as an intermediate in the production of pharmaceuticals, pesticides, and other agrochemicals, where its boiling point is a critical factor in process design and optimization.
- Analytical Chemistry: The boiling point of triethylamine is an important parameter in analytical techniques, such as gas chromatography, where it is used as a reference compound or internal standard.
Numerical Examples and Calculations
To illustrate the practical application of the boiling point of triethylamine, let’s consider the following examples:
Example 1: Vapor Pressure Calculation
Given the enthalpy of vaporization (ΔHvap) of triethylamine as 33.9 kJ/mol, calculate the vapor pressure of the compound at 25 °C.
Using the Clausius-Clapeyron equation:
ln(P) = (-ΔHvap/R)(1/T) + C
ln(P) = (-33.9 × 10^3 J/mol) / (8.314 J/mol·K) × (1 / (25 + 273.15 K)) + C
ln(P) = -3.89 + C
P = e^(-3.89) = 0.0203 kPa
Therefore, the vapor pressure of triethylamine at 25 °C is approximately 0.0203 kPa.
Example 2: Boiling Point Estimation
Estimate the boiling point of triethylamine at an atmospheric pressure of 80 kPa.
Using the Clausius-Clapeyron equation and the known boiling point of 88.6 °C (361.9 K) at 101.3 kPa:
ln(P1/P2) = (-ΔHvap/R)(1/T1 - 1/T2)
ln(101.3 kPa / 80 kPa) = (-33.9 × 10^3 J/mol) / (8.314 J/mol·K) × (1/361.9 K - 1/T2)
T2 = 366.4 K = 93.2 °C
Therefore, the estimated boiling point of triethylamine at an atmospheric pressure of 80 kPa is 93.2 °C.
Conclusion
The boiling point of triethylamine is a crucial parameter in various chemical and industrial applications. This comprehensive guide has provided a detailed understanding of the factors influencing the boiling point, the experimental determination methods, and the practical implications of this property. By delving into the theoretical considerations, numerical examples, and specific details, this article aims to serve as a valuable resource for science students and professionals working with triethylamine and related compounds.
References:
- Lide, D. R. (Ed.). (2005). CRC Handbook of Chemistry and Physics (86th ed.). CRC Press.
- Yaws, C. L. (2015). Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds. Knovel.
- Riddick, J. A., Bunger, W. B., & Sakano, T. K. (1986). Organic Solvents: Physical Properties and Methods of Purification (4th ed.). Wiley-Interscience.
- Sigma-Aldrich. (n.d.). Triethylamine. Retrieved from https://www.sigmaaldrich.com/US/en/product/aldrich/471283
- PubChem. (n.d.). Triethylamine. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Triethylamine

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.