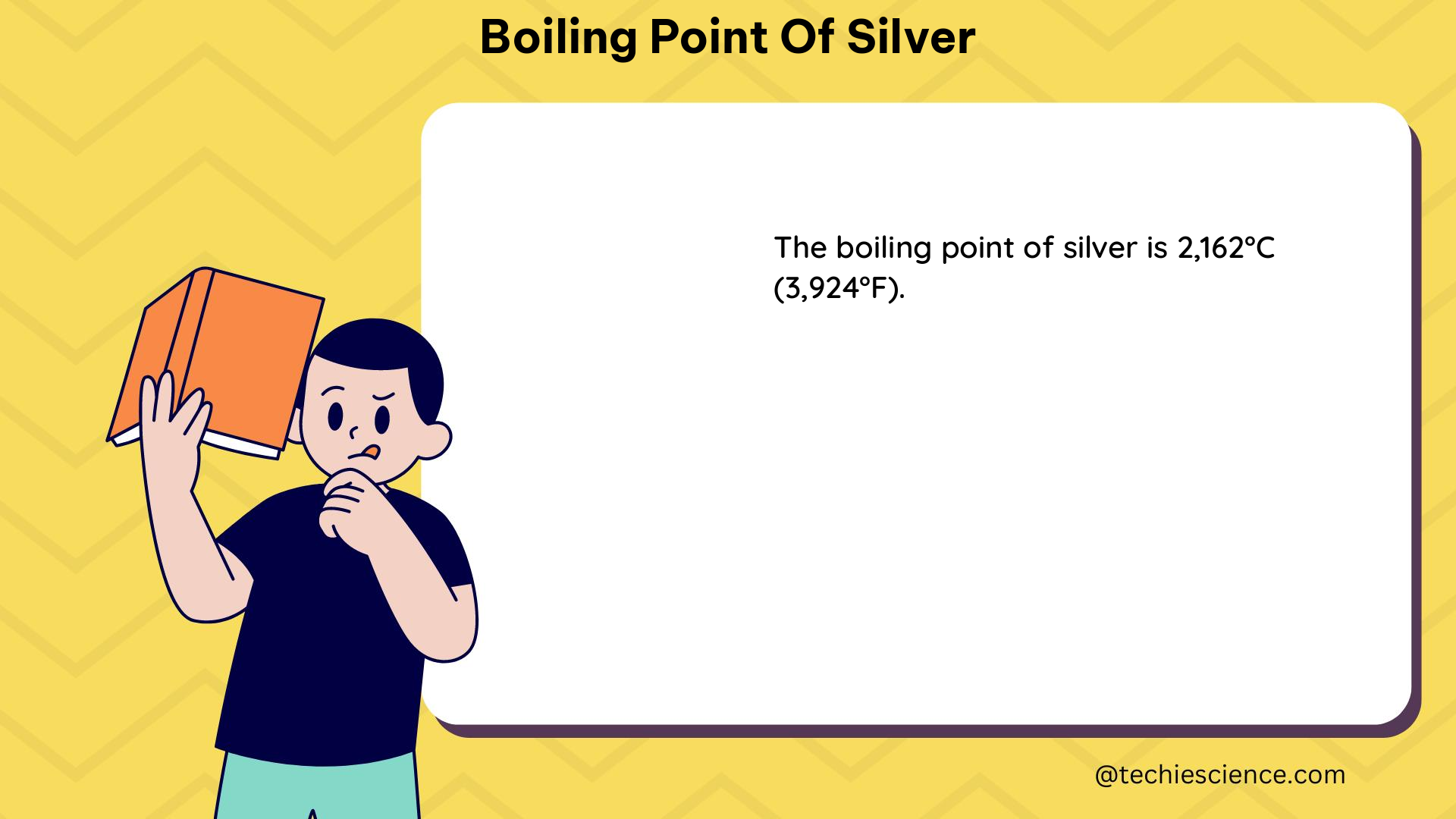
The boiling point of silver is a crucial physical property that plays a significant role in various industrial and scientific applications. At 2162 °C (3923.6 °F or 2435 K), the boiling point of silver is a fixed point in the International Temperature Scale of 1990 (ITS-90), which serves as the standard for temperature measurement.
Understanding the Boiling Point of Silver
The boiling point of silver is the temperature at which the vapor pressure of the liquid silver equals the standard atmospheric pressure of 101.3 kilopascals (kPa). At this temperature, the silver undergoes a phase change from a liquid to a gaseous state, and its physical and chemical properties undergo significant transformations.
The boiling point of silver is a crucial parameter in various industries, including electronics, photography, and the medical field, where precise temperature control and measurement are essential.
Measuring the Boiling Point of Silver
To accurately measure the boiling point of silver, researchers and scientists employ specialized apparatus and methods that can provide reliable and reproducible results. One such method involves the use of a thermocouple or a resistance temperature detector (RTD) to measure the temperature of the silver vapor in contact with the sensor.
Thermocouple Measurement
A thermocouple is a device that generates a voltage proportional to the temperature difference between two junctions. By placing the thermocouple in direct contact with the silver vapor, the temperature of the boiling silver can be measured accurately. The thermocouple is then connected to a data logger or a computer, which records and displays the temperature as a function of time.
Resistance Temperature Detector (RTD) Measurement
An RTD is a device that measures temperature by correlating the resistance of a metal (typically platinum) with temperature. Similar to the thermocouple method, the RTD is placed in the silver vapor, and the resistance is measured and converted to temperature using a calibration curve.
Apparatus Calibration
Before conducting the boiling point measurement, the apparatus must be calibrated to ensure its accuracy and precision. This process involves comparing the measured temperature with a known reference standard, such as the melting point of a pure metal or the triple point of water, to ensure the reliability of the results.
Factors Affecting the Boiling Point of Silver
The boiling point of silver can be influenced by various factors, including:
- Pressure: The boiling point of silver is directly related to the surrounding pressure. As the pressure increases, the boiling point also increases, and vice versa. This relationship is described by the Clausius-Clapeyron equation:
ln(P2/P1) = (L/R)[(1/T1) - (1/T2)]
where:
– P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively
– L is the latent heat of vaporization
– R is the universal gas constant
-
Impurities: The presence of impurities in the silver can affect its boiling point. Impurities can alter the vapor pressure of the liquid silver, leading to a change in the boiling point.
-
Surface Tension: The surface tension of the liquid silver can also influence its boiling point. As the surface tension increases, the boiling point may slightly increase as well.
-
Gravitational Effects: In microgravity or low-gravity environments, the boiling point of silver may be affected due to changes in the behavior of the liquid-vapor interface and the formation of bubbles.
Applications of the Boiling Point of Silver
The boiling point of silver is an essential parameter in various applications, including:
-
Temperature Measurement and Calibration: The boiling point of silver is a fixed point in the ITS-90 scale, making it a valuable reference for temperature measurement and calibration of instruments.
-
Metallurgy and Material Processing: The boiling point of silver is crucial in the production, processing, and handling of silver-based materials, ensuring proper temperature control and phase transitions.
-
Electronics and Semiconductor Industry: Silver is widely used in electronic components, and its boiling point is a critical factor in the design and manufacturing of these devices.
-
Photography and Imaging: Silver-based compounds are used in photographic and imaging processes, where the boiling point of silver plays a role in the development and processing of these materials.
-
Medical and Dental Applications: Silver and silver-containing compounds are used in various medical and dental applications, and the boiling point of silver is an important consideration in these fields.
Conclusion
The boiling point of silver, a fixed point in the ITS-90 scale, is a crucial physical property that has far-reaching implications in various industries and scientific applications. By understanding the factors that influence the boiling point of silver and the methods used to measure it, researchers and scientists can optimize processes, ensure accurate temperature control, and push the boundaries of technological innovation.
References:
- Technical data for the element Silver in the Periodic Table. Periodic Table. https://periodictable.com/Elements/047/data.html
- Boiling Point Measurement. Analytical Chemistry Wiki. https://chem.libretexts.org/Courses/University_of_California_Davis/Analytical_Chemistry_2A_(Green_Book)/03%3A_Thermodynamics/3.04%3A_Boiling_Point_Measurement
- Boiling Point of Silver. Engineering ToolBox. https://www.engineeringtoolbox.com/boiling-points-d_1185.html
- Silver (Ag) – Properties, Structure, and Applications. ScienceStruck. https://sciencestruck.com/silver-properties-structure-and-applications
- Clausius-Clapeyron equation. Wikipedia. https://en.wikipedia.org/wiki/Clausius%E2%80%93Clapeyron_relation

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.