The boiling point of phenol, a widely used organic compound, is a crucial property that has been extensively studied and documented. This comprehensive guide delves into the intricacies of the boiling point of phenol, providing a wealth of technical details, formulas, and practical applications to aid science students in their understanding of this fundamental concept.
Understanding the Boiling Point of Phenol
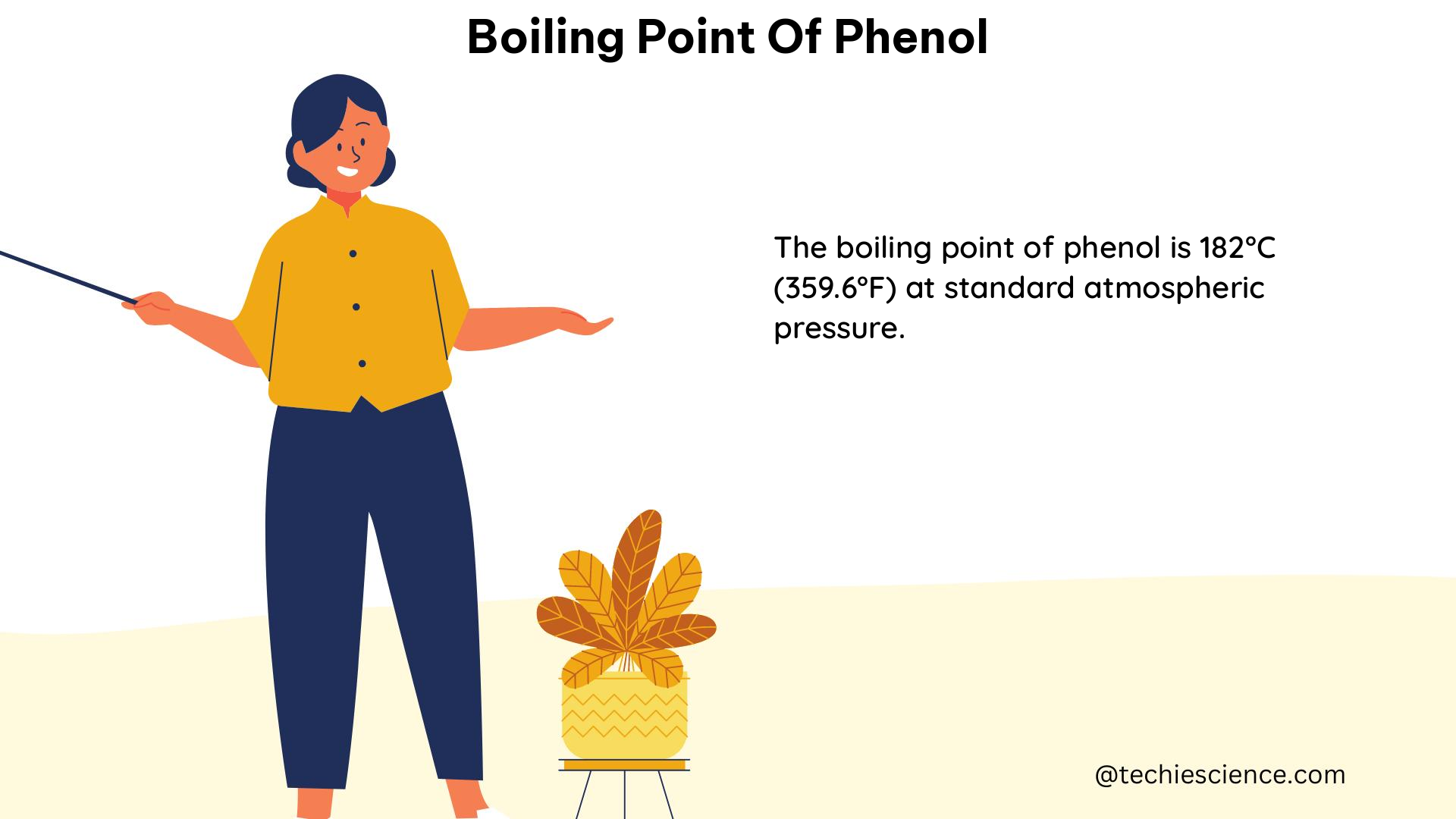
The boiling point of phenol, as determined through various experiments and studies, is 360 degrees Fahrenheit (182.2 degrees Celsius) at 760 millimeters of mercury (mmHg). This value is based on the data provided by the National Toxicology Program (NTP) in 1992.
Factors Influencing the Boiling Point
The boiling point of a substance is influenced by several factors, including:
-
Intermolecular Forces: The strength of the intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces, plays a significant role in determining the boiling point.
-
Molecular Size and Weight: Larger and heavier molecules generally have higher boiling points due to the increased London dispersion forces.
-
Pressure: The boiling point of a substance is directly proportional to the surrounding pressure. As the pressure increases, the boiling point also increases, as described by the Clausius-Clapeyron equation:
ln(P2/P1) = (ΔHvap/R) * (1/T1 - 1/T2)
Where:
– P1 and P2 are the pressures at temperatures T1 and T2, respectively.
– ΔHvap is the enthalpy of vaporization.
– R is the universal gas constant.
Comparison with Ethanol
Interestingly, the boiling point of phenol (182.2°C) is higher than that of ethanol (78.3°C), despite both compounds being able to form hydrogen bonds and dipole-dipole interactions. This difference can be attributed to the larger London dispersion forces (LDF) in phenol due to its larger molecular size and weight.
The London dispersion forces are temporary dipole-dipole interactions that arise from the fluctuations in the electron density of a molecule. As the molecular size and weight increase, the London dispersion forces become more significant, leading to a higher boiling point.
Predicting the Boiling Point of Phenol
In addition to the experimental determination of the boiling point, researchers have also explored methods to predict the boiling point of phenol using various approaches.
Topological Indices
One such approach involves the use of topological indices, which are numerical values that describe the molecular structure of a compound. A study published in 2018 used quantitative correlations between various molecular properties and chemical structures to predict the normal boiling points and enthalpy of vaporization of alcohols and phenols.
The study employed several topological indices, such as the Wiener index, Balaban index, and Randić index, to develop predictive models for the boiling points of these compounds. The results demonstrated the effectiveness of using topological indices in estimating the boiling points of phenols and alcohols.
Quantum Chemical Calculations
Another method for predicting the boiling point of phenol involves the use of quantum chemical calculations. These calculations, based on the principles of quantum mechanics, can provide insights into the electronic structure and intermolecular interactions of the compound.
By applying computational chemistry techniques, such as density functional theory (DFT) or ab initio methods, researchers can estimate the thermodynamic properties, including the boiling point, of phenol and other organic compounds. These computational approaches can be particularly useful when experimental data is limited or difficult to obtain.
Practical Applications of the Boiling Point of Phenol
The boiling point of phenol has various practical applications in different fields, including:
-
Chemical Processing: The boiling point of phenol is an essential parameter in the design and optimization of chemical processes, such as distillation, evaporation, and drying, where the phase change from liquid to vapor is a critical step.
-
Environmental Monitoring: Phenol is a common environmental pollutant, and its boiling point is used in the development of analytical methods for the detection and quantification of phenol in air, water, and soil samples.
-
Pharmaceutical and Cosmetic Industries: The boiling point of phenol is relevant in the formulation and production of various pharmaceutical and cosmetic products, where phenol may be used as a preservative or active ingredient.
-
Material Science: The boiling point of phenol is a crucial property in the synthesis and characterization of phenol-based polymers and resins, which have numerous applications in the materials science and engineering fields.
-
Safety and Handling: The boiling point of phenol is an important consideration in the safe handling and storage of this compound, as it can pose health and environmental risks if not properly managed.
Conclusion
The boiling point of phenol is a well-defined and measurable property that has been extensively studied and documented. This comprehensive guide has provided a wealth of technical details, formulas, and practical applications to aid science students in their understanding of this fundamental concept.
By exploring the factors influencing the boiling point, the comparison with ethanol, and the methods for predicting the boiling point, this guide has aimed to equip students with a deep understanding of the boiling point of phenol and its significance in various scientific and industrial applications.
References
- Chemeo. (n.d.). Phenol, 2-methyl-. Retrieved from https://www.chemeo.com/cid/17-372-0/Phenol%2C%202-methyl-
- Reddit. (2022). Why does phenol have a higher melting/boiling point than ethanol? Retrieved from https://www.reddit.com/r/Mcat/comments/uoc435/why_does_phenol_have_a_higher_meltingboiling/
- PubChem. (n.d.). Phenol. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Phenol
- Shahsavari, H., Shahsavari, V., & Shahsavari, S. (2018). Prediction of the Normal Boiling Points and Enthalpy of Vaporizations of Alcohols and Phenols Using Topological Indices. Journal of Chemical & Engineering Data, 63(8), 2893-2902. Retrieved from https://www.researchgate.net/publication/326247608_Prediction_of_the_Normal_Boiling_Points_and_Enthalpy_of_Vaporizations_of_Alcohols_and_Phenols_Using_Topological_Indices

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.