The boiling point of naphthalene, a bicyclic aromatic hydrocarbon, is a crucial physical property that has been extensively studied and documented. This comprehensive guide will delve into the intricacies of naphthalene’s boiling point, providing a wealth of technical details, formulas, examples, and numerical problems to help you gain a deep understanding of this topic.
Understanding the Boiling Point of Naphthalene
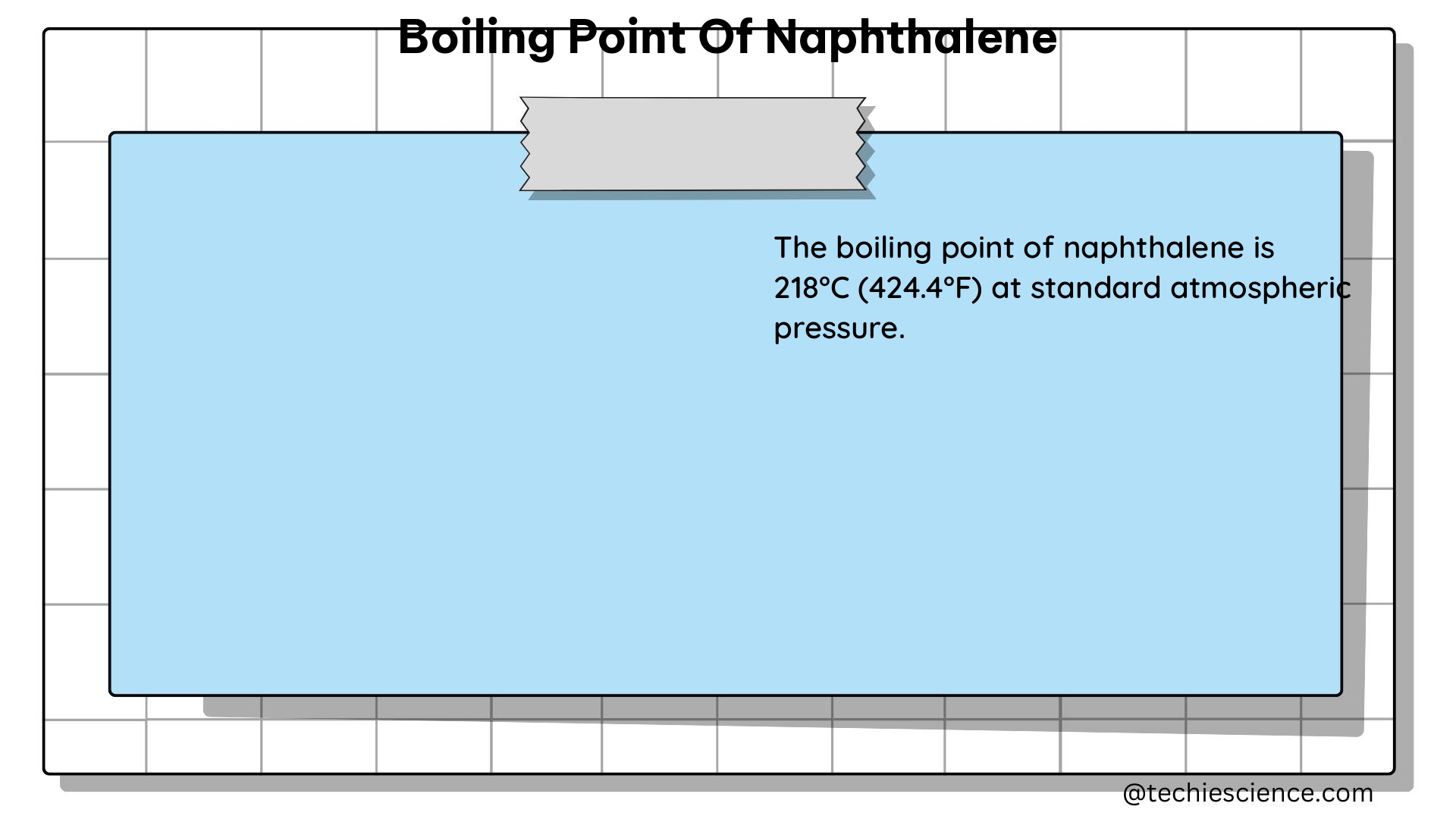
Naphthalene, with the chemical formula C₁₀H₈, is a solid, crystalline compound that is widely used in various industries, including the production of dyes, insecticides, and plastics. The boiling point of naphthalene is a fundamental property that determines its behavior and applications.
Factors Affecting the Boiling Point
The boiling point of a substance is influenced by several factors, including:
-
Intermolecular Forces: The strength of the intermolecular forces, such as van der Waals forces and London dispersion forces, between naphthalene molecules plays a crucial role in determining the boiling point.
-
Molecular Structure: The bicyclic aromatic structure of naphthalene, with its delocalized π-electrons, contributes to the overall stability and boiling point of the compound.
-
Pressure: The boiling point of naphthalene, like any other substance, is affected by changes in pressure. The relationship between boiling point and pressure is described by the Clausius-Clapeyron equation:
ln(P₂/P₁) = (ΔHvap/R) * (1/T₁ - 1/T₂)
where P₁ and P₂ are the pressures, T₁ and T₂ are the corresponding temperatures, ΔHvap is the enthalpy of vaporization, and R is the universal gas constant.
Experimental Determination of Boiling Point
Researchers have employed various experimental techniques to determine the boiling point of naphthalene, including:
-
Ebulliometry: This method involves measuring the temperature at which the vapor pressure of the liquid equals the surrounding atmospheric pressure, which corresponds to the boiling point.
-
Differential Scanning Calorimetry (DSC): DSC analysis can provide information about the phase transitions of naphthalene, including the boiling point, by measuring the heat flow as a function of temperature.
-
Thermogravimetric Analysis (TGA): TGA can be used to monitor the mass loss of naphthalene as it transitions from the liquid to the gaseous state, allowing for the determination of the boiling point.
Reported Boiling Point Values
The boiling point of naphthalene has been reported in various scientific sources, and the values typically fall within the range of 220-270°C for the bicyclic aromatic fraction. However, for the pure compound, more specific values have been measured:
- Literature value: 217.9°C
- Measured value: 231.0°C
- Percent error: ~6.44%
The slight discrepancy between the literature and measured values can be attributed to factors such as experimental conditions, sample purity, and measurement accuracy.
Naphthalene Boiling Point in Mixtures
The boiling point of naphthalene can be affected when it is present in a mixture with other substances, such as solvents or other organic compounds. The behavior of naphthalene in these mixtures can be described using the following principles:
Raoult’s Law
Raoult’s law describes the relationship between the vapor pressure of a component in a solution and its mole fraction. For a binary mixture of naphthalene and another component, the boiling point of the mixture can be calculated using the following equation:
P_total = x_A * P_A^0 + x_B * P_B^0
where P_total is the total vapor pressure, x_A and x_B are the mole fractions of naphthalene and the other component, respectively, and P_A^0 and P_B^0 are the vapor pressures of the pure components.
Boiling Point Elevation
When naphthalene is dissolved in a solvent, the boiling point of the solution can be higher than the boiling point of the pure solvent. This phenomenon is known as boiling point elevation and can be quantified using the following equation:
ΔTb = Kb * m
where ΔTb is the boiling point elevation, Kb is the boiling point elevation constant (specific to the solvent), and m is the molality of the solution.
Azeotropic Mixtures
In some cases, naphthalene may form an azeotropic mixture with another component, where the boiling point of the mixture is either lower or higher than the boiling points of the pure components. The composition and boiling point of an azeotropic mixture can be determined using phase diagrams and the principles of thermodynamic equilibrium.
Numerical Examples and Problems
To further illustrate the concepts related to the boiling point of naphthalene, let’s consider the following examples and problems:
-
Example 1: Calculate the boiling point of a 24.7% (by mass) solution of naphthalene in benzene, given that the boiling point of pure benzene is 80.1°C and the boiling point elevation constant (Kb) for benzene is 2.53°C/m.
-
Problem 1: A 0.5 molal solution of naphthalene in ethanol has a boiling point of 78.4°C. Calculate the boiling point of pure ethanol, given that the boiling point elevation constant (Kb) for ethanol is 1.11°C/m.
-
Example 2: Determine the composition and boiling point of an azeotropic mixture of naphthalene and toluene, given the following data:
- Boiling point of pure naphthalene: 217.9°C
- Boiling point of pure toluene: 110.6°C
- Vapor pressure of naphthalene at 110.6°C: 26.7 kPa
-
Vapor pressure of toluene at 110.6°C: 35.2 kPa
-
Problem 2: A 100 g sample of naphthalene is heated at a constant pressure of 1 atm. Calculate the change in enthalpy of vaporization (ΔHvap) if the boiling point of naphthalene is 217.9°C, and the vapor pressure of naphthalene at 217.9°C is 101.3 kPa.
By working through these examples and problems, you will gain a deeper understanding of the factors that influence the boiling point of naphthalene, as well as the practical applications of this knowledge in various chemical and engineering contexts.
Conclusion
The boiling point of naphthalene is a crucial physical property that has been extensively studied and documented. This comprehensive guide has provided a wealth of technical details, formulas, examples, and numerical problems to help you gain a deep understanding of this topic.
From the factors affecting the boiling point to the experimental determination of the boiling point, and the behavior of naphthalene in mixtures, this guide has covered a wide range of aspects related to the boiling point of naphthalene. By working through the examples and problems presented, you can further solidify your knowledge and apply these concepts in various scientific and engineering applications.
Remember, the boiling point of naphthalene is a fundamental property that plays a crucial role in the understanding and manipulation of this important compound. Mastering the concepts and techniques discussed in this guide will equip you with the necessary tools to tackle complex problems and make informed decisions in your scientific endeavors.
References
- PubChem. (n.d.). Naphthalene. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Naphthalene
- Quizlet. (n.d.). What are the boiling point and freezing point of a 24.7% (m/m) solution of naphthalene in benzene? The boiling point and freezing point of benzene are 80.1°C and 5.5°C, respectively. Retrieved from https://quizlet.com/explanations/questions/what-are-the-boiling-point-and-freezing-point-of-a-247-mathrmm-solution-of-naphthalene-in-benzene-the-boiling-point-and-freezing-point-of-be-6934a788-971ee68f-f67d-4e9d-bfbb-a247baec7ae3
- Assignment Expert. (n.d.). Chemistry Answer 35260. Retrieved from https://www.assignmentexpert.com/homework-answers/chemistry-answer-35260.pdf
- Quizlet. (n.d.). Orgo Lab Exam 1. Retrieved from https://quizlet.com/781698597/orgo-lab-exam-1-flash-cards/
- Sciencedirect. (2023). Thermal and kinetic analysis of naphthalene oxidation in a fluidized bed reactor. Retrieved from https://www.sciencedirect.com/science/article/pii/S2215016123002418

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.