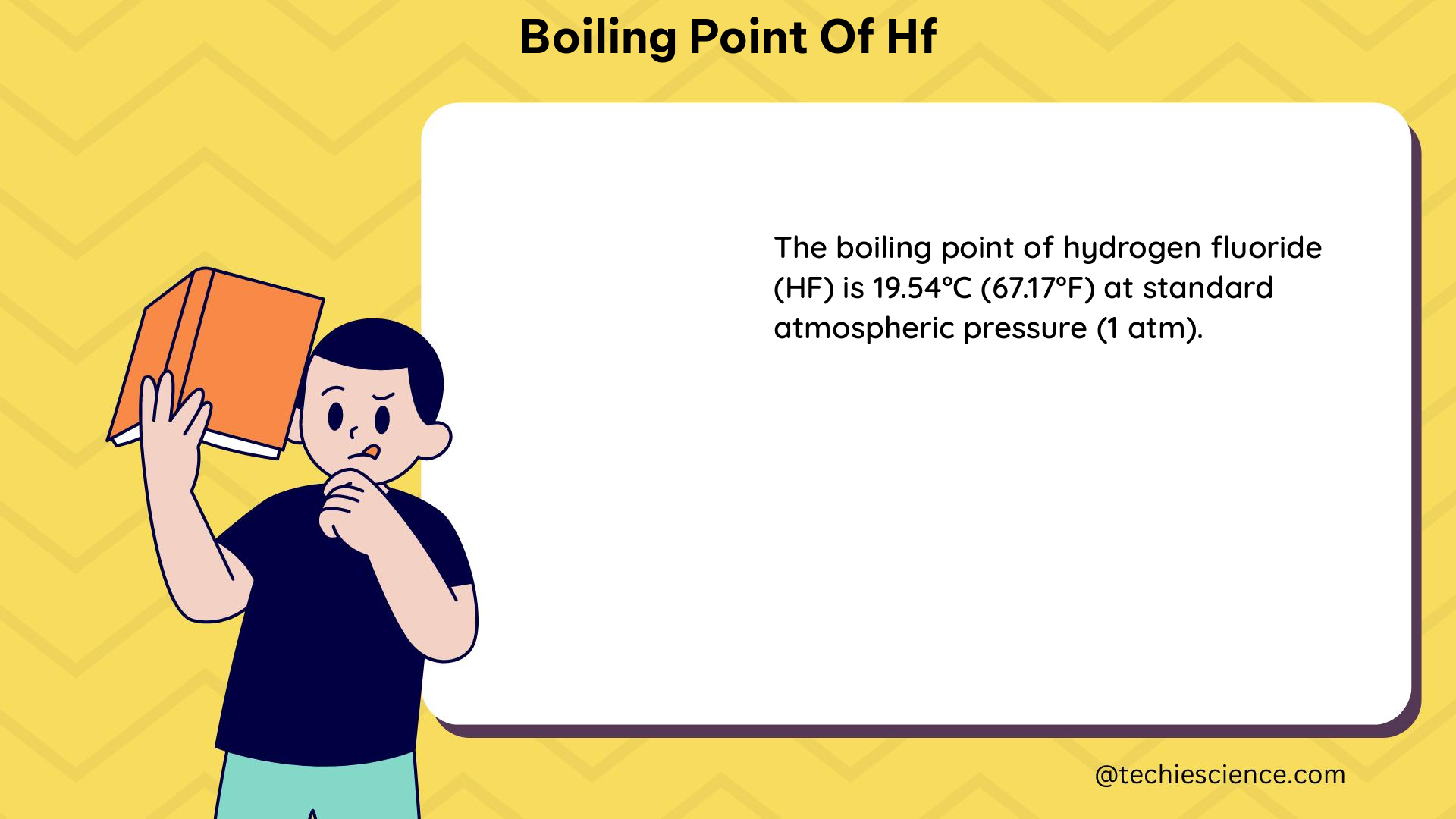
The normal boiling point of Hydrogen Fluoride (HF) is 19.5°C or 292.65 Kelvin, which is relatively high compared to other hydrogen halides due to the presence of strong intermolecular hydrogen bonding. This comprehensive guide delves into the intricacies of HF’s boiling point, providing a wealth of technical details and practical insights for science students and enthusiasts.
Understanding Hydrogen Bonding in HF
Hydrogen bonding is a unique type of intermolecular force that occurs when a hydrogen atom, covalently bonded to a highly electronegative element (such as fluorine in HF), interacts with another highly electronegative atom (such as the fluorine in an adjacent HF molecule). This interaction creates a partially positively charged hydrogen atom and a partially negatively charged electronegative atom, resulting in a strong electrostatic attraction.
The strength of hydrogen bonding in HF can be quantified using the following equation:
E_HB = -0.2 × (χ_H - χ_F)^2
Where:
– E_HB is the hydrogen bonding energy in kJ/mol
– χ_H is the electronegativity of hydrogen (2.20)
– χ_F is the electronegativity of fluorine (3.98)
Plugging in the values, we get:
E_HB = -0.2 × (2.20 - 3.98)^2 = -19.84 kJ/mol
This high hydrogen bonding energy of -19.84 kJ/mol in HF, compared to other hydrogen halides, is a key factor in its elevated boiling point.
Comparing Boiling Points of Hydrogen Halides
To better understand the boiling point of HF, it is helpful to compare it with the boiling points of other hydrogen halides, such as HCl and HI. The table below summarizes the boiling points of these compounds:
| Compound | Boiling Point (°C) |
|---|---|
| HF | 19.5 |
| HCl | -85.0 |
| HBr | -66.8 |
| HI | -35.5 |
As shown, the boiling point of HF is significantly higher than the other hydrogen halides, despite having the lowest molecular mass. This can be attributed to the strong hydrogen bonding present in HF, which requires more energy to overcome compared to the weaker London dispersion forces in HCl and HI.
Factors Affecting the Boiling Point of HF
The boiling point of HF is influenced by several factors, including:
-
Molecular Mass: While molecular mass generally correlates with boiling point, the presence of strong hydrogen bonding in HF overrides this trend, leading to a higher boiling point than expected based on its molecular mass alone.
-
Electronegativity Difference: The large electronegativity difference between hydrogen (2.20) and fluorine (3.98) in HF contributes to the strength of the hydrogen bonding, which in turn raises the boiling point.
-
Hydrogen Bonding Network: HF molecules can form an extensive network of hydrogen bonds, creating a more cohesive liquid phase that requires more energy to overcome, resulting in a higher boiling point.
-
Molecular Geometry: The linear molecular geometry of HF, with the highly electronegative fluorine atoms positioned on either side of the hydrogen atom, facilitates the formation of strong intermolecular hydrogen bonds.
-
Pressure: The boiling point of HF, like any substance, is also influenced by changes in pressure. As pressure increases, the boiling point of HF increases, as described by the Clausius-Clapeyron equation:
ln(P2/P1) = (ΔHvap/R) × (1/T1 - 1/T2)
Where:
– P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively
– ΔHvap is the enthalpy of vaporization
– R is the universal gas constant
Practical Applications of HF’s Boiling Point
The high boiling point of HF has several practical applications in various fields:
-
Refrigeration: HF has been used as a refrigerant in the past, taking advantage of its high boiling point compared to other halides.
-
Chemical Reactions: The elevated boiling point of HF allows for more controlled and efficient chemical reactions, as the compound can be easily vaporized and condensed as needed.
-
Etching and Cleaning: HF’s high boiling point makes it suitable for use in etching and cleaning processes, particularly in the semiconductor industry, where it is used to etch silicon and remove metal oxides.
-
Analytical Chemistry: The boiling point of HF is a crucial parameter in analytical techniques, such as gas chromatography, where it is used to separate and identify chemical compounds.
-
Safety Considerations: The high boiling point of HF also has important safety implications, as it can lead to the formation of concentrated HF solutions, which are highly corrosive and toxic. Proper handling and containment procedures are essential when working with HF.
Conclusion
The boiling point of Hydrogen Fluoride (HF) is a fascinating and complex topic, with its elevated value of 19.5°C or 292.65 Kelvin being primarily attributed to the strong intermolecular hydrogen bonding present in the compound. By understanding the factors that influence HF’s boiling point, such as molecular mass, electronegativity, hydrogen bonding, and pressure, we can gain valuable insights into the behavior and applications of this unique chemical substance.
References
- Socratic. (2017-04-22). Why are the normal boiling points of HF, and H2O so high? Retrieved from https://socratic.org/questions/58f9d3e2b72cff1a4427435a
- Chem.LibreTexts. (2023-07-07). Real Gases – Deviations from Ideal Behavior. Retrieved from https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:Chemistry–The_Central_Science(Brown_et_al.)/10:Gases/10.09:_Real_Gases-_Deviations_from_Ideal_Behavior
- ResearchGate. (2010-10-08). A Closer Look at Trends in Boiling Points of Hydrides. Retrieved from https://www.researchgate.net/publication/231268906_A_Closer_Look_at_Trends_in_Boiling_Points_of_Hydrides
- Chemistry Stack Exchange. (2017-11-28). Boiling points of HF, HCl, HI. Retrieved from https://chemistry.stackexchange.com/questions/86520/boiling-points-of-hf-hcl-hi
- SETAC. (2009-11-06). Quantitative structure‐property relationships for prediction of boiling points, vapor pressures, and melting points. Retrieved from https://setac.onlinelibrary.wiley.com/doi/full/10.1897/01-363

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.