The boiling point of heptane, a saturated aliphatic hydrocarbon with the chemical formula C7H16, is a crucial physical property that has been extensively studied and documented. This comprehensive guide will delve into the intricacies of heptane’s boiling point, providing a wealth of technical details, formulas, and practical applications for science students and enthusiasts.
Understanding the Boiling Point of Heptane
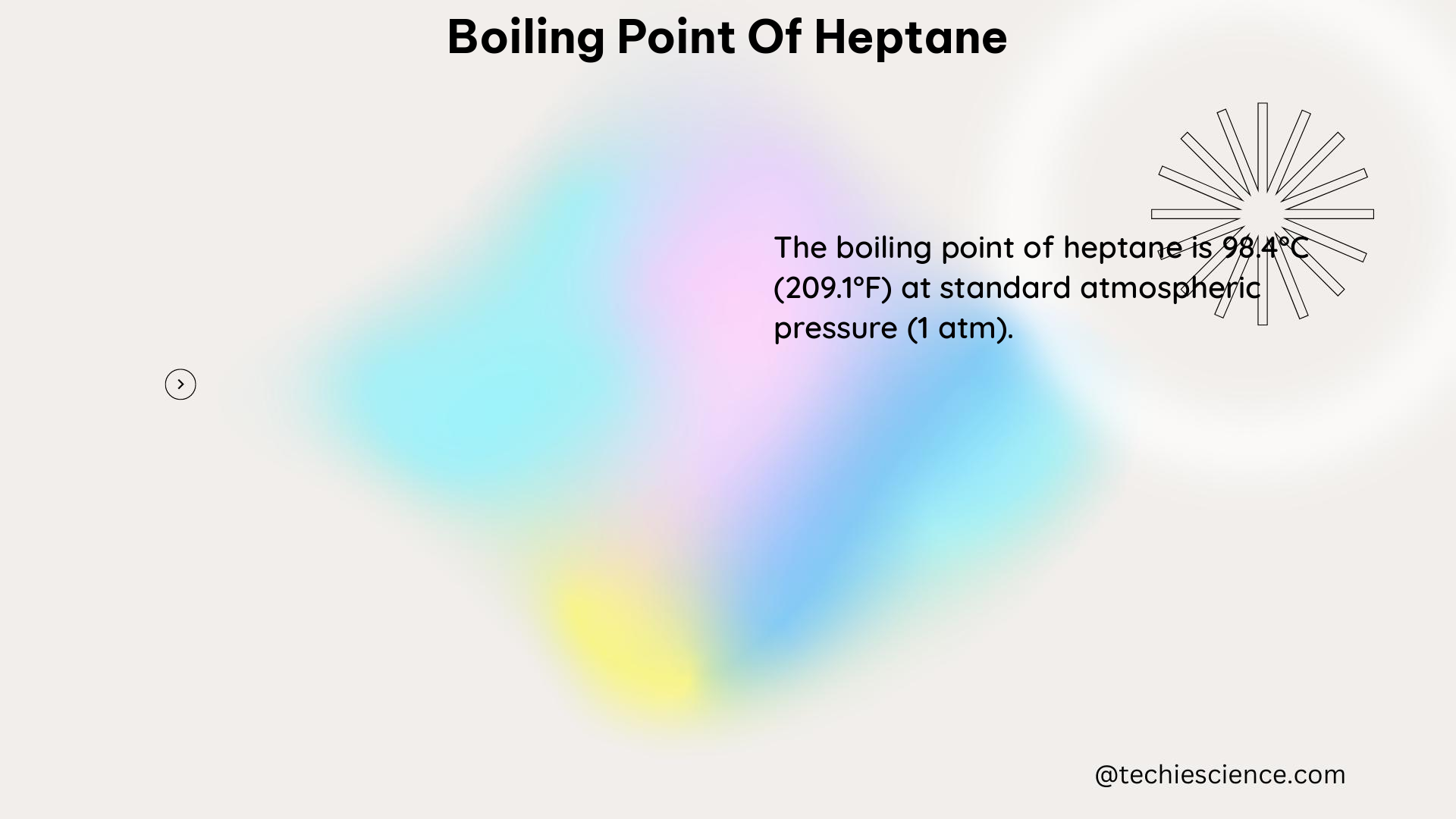
The boiling point of heptane, as mentioned in the initial answer, is 209.2°F (98.42°C) at standard atmospheric pressure (1 atm or 101.3 kPa or 760 mmHg). This temperature represents the point at which the vapor pressure of the liquid heptane equals the surrounding atmospheric pressure, allowing the liquid to transition into a gaseous state.
Factors Affecting the Boiling Point
The boiling point of heptane, like any other substance, is influenced by various factors, including:
- Pressure: As the pressure decreases, the boiling point of heptane also decreases. This relationship can be described by the Clausius-Clapeyron equation:
ln(P2/P1) = (ΔHvap/R) * (1/T1 - 1/T2)
Where:
– P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively
– ΔHvap is the enthalpy of vaporization of heptane
– R is the universal gas constant
-
Intermolecular Forces: The strength of intermolecular forces, such as van der Waals forces, between heptane molecules can affect the boiling point. Stronger intermolecular forces generally result in a higher boiling point.
-
Molecular Structure: The linear, non-branched structure of heptane contributes to its relatively high boiling point compared to other alkanes of similar molecular weight.
-
Impurities: The presence of impurities in heptane can alter its boiling point, either increasing or decreasing it depending on the nature of the impurities.
Experimental Measurements of Boiling Point
Numerous studies have been conducted to measure the boiling point of heptane under various conditions. Some notable examples include:
-
Smith (1940): Measured the boiling point of heptane over a range of pressures from 100 to 1,500 millimeters of mercury (mmHg). The results showed a decrease in boiling point as the pressure decreased.
-
Stephenson and Malanowski (1987): Reported the boiling point of heptane at different temperatures and pressures, as well as the enthalpy of vaporization and other thermodynamic properties.
-
NASA Technical Reports Server (2004): Investigated the combustion behavior of heptane droplets, which included measurements of the boiling point under different conditions.
These studies, along with others, have contributed to the comprehensive understanding of heptane’s boiling point and its behavior under various experimental conditions.
Heptane Boiling Point in Mixtures
The boiling point of heptane can be further influenced by its presence in mixtures with other substances. Researchers have studied the behavior of heptane in various binary and ternary mixtures, such as:
-
Heptane-Hexane Mixtures: The boiling point of heptane-hexane mixtures can deviate from the ideal behavior due to the formation of azeotropes, which are mixtures with a constant boiling point.
-
Heptane-Octane Mixtures: The boiling point of heptane-octane mixtures can also exhibit non-ideal behavior, with the formation of azeotropes and the presence of excess Gibbs free energy.
-
Heptane-Cyclohexanol Mixtures: The addition of cyclohexanol to heptane can affect the vapor pressures and boiling points of the mixture, as well as the thermodynamic properties.
These studies on heptane-containing mixtures provide valuable insights into the complex behavior of heptane and its interactions with other substances.
Applications and Practical Considerations
The boiling point of heptane is an important property in various applications, including:
-
Fuel and Solvent Applications: Heptane is used as a fuel component in gasoline and as a solvent in various industrial processes. The boiling point of heptane is a crucial parameter in the design and optimization of these applications.
-
Combustion and Droplet Studies: The boiling point of heptane is a key factor in understanding the combustion behavior of heptane-based fuels, particularly in the context of droplet combustion studies.
-
Thermodynamic Modeling: The boiling point of heptane, along with other thermodynamic properties, is essential for the development of accurate models and simulations in various fields, such as chemical engineering and thermodynamics.
-
Environmental Considerations: Heptane is a volatile organic compound (VOC) and its boiling point is relevant in understanding its environmental behavior, such as evaporation rates and potential for atmospheric pollution.
In summary, the boiling point of heptane is a well-studied and well-documented physical property that has significant implications in various scientific and industrial applications. This comprehensive guide has provided a detailed overview of the factors affecting the boiling point, experimental measurements, and the behavior of heptane in mixtures, equipping science students and enthusiasts with a deeper understanding of this important property.
References:
- Boiling point of heptane. (n.d.). In Wikipedia. Retrieved June 17, 2024, from https://en.wikipedia.org/wiki/Boiling_point_of_heptane
- Heptane – the NIST WebBook. (n.d.). In NIST. Retrieved June 17, 2024, from https://webbook.nist.gov/cgi/cbook.cgi?ID=C142825&Mask=4
- A Treatment of Measurements of Heptane Droplet Combustion … (n.d.). In NASA Technical Reports Server. Retrieved June 17, 2024, from https://ntrs.nasa.gov/api/citations/20040000941/downloads/20040000941.pdf
- Smith, J. M. (1940). The Vapor Pressures of Pure Substances. Industrial & Engineering Chemistry, 32(6), 761–764. https://doi.org/10.1021/ie50366a007
- Stephenson, R. M., & Malanowski, S. (1987). Handbook of the Thermodynamics of Organic Compounds. Springer Netherlands. https://doi.org/10.1007/978-94-009-3969-8

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.