The boiling point of gasoline is a critical quality specification that is determined through various methods, including atmospheric distillation and simulated distillation using gas chromatography. The boiling point distribution of gasoline provides important information for refinery operation, including the potential mass percent yield of products and the amount of residue produced, which is crucial for determining the economics of the refining process.
Understanding the Boiling Point of Gasoline
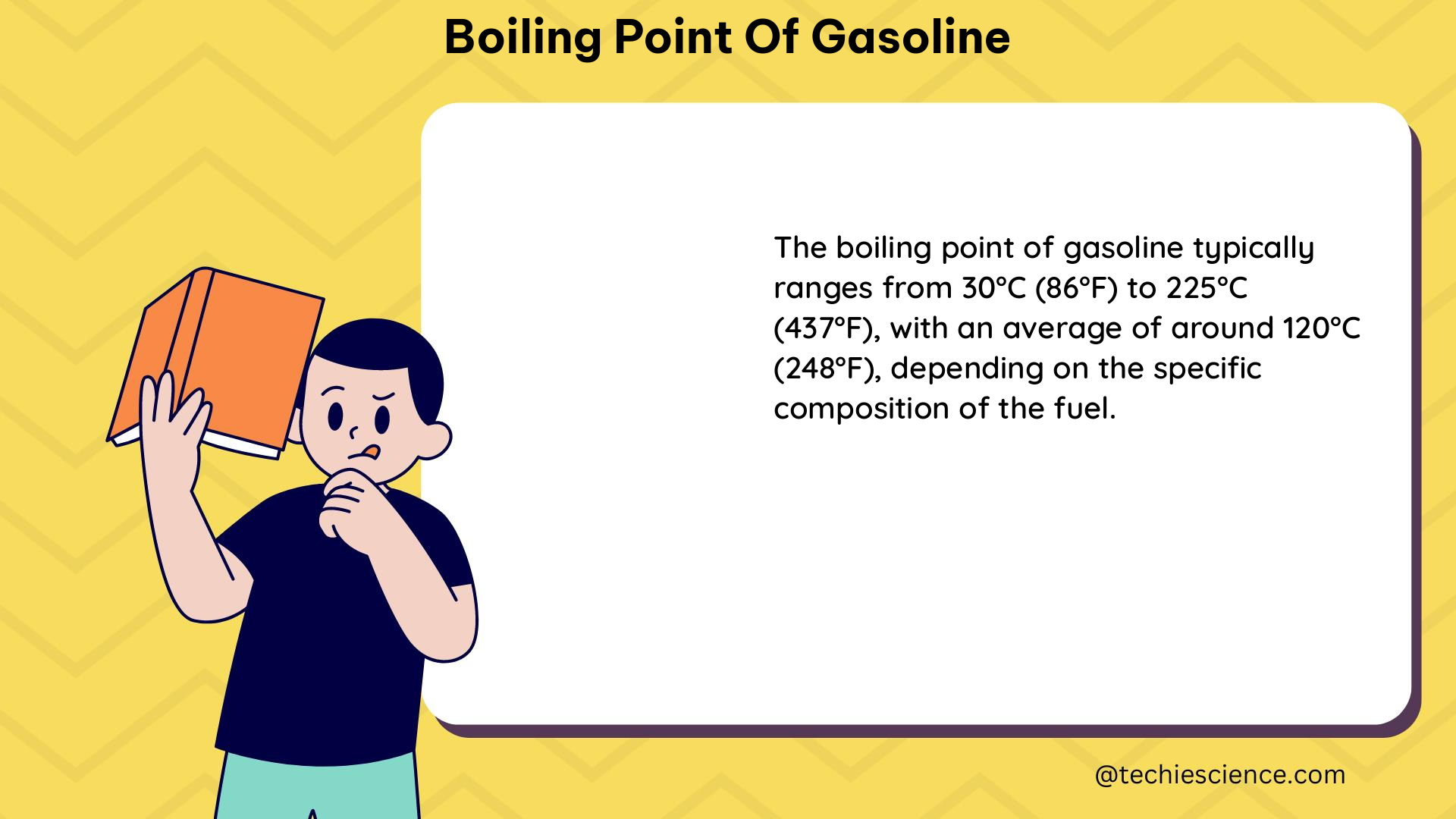
Gasoline is a complex mixture of hydrocarbons, primarily consisting of alkanes, alkenes, and aromatic compounds. The boiling point of gasoline is influenced by the composition and relative proportions of these various hydrocarbon components. The boiling point range of gasoline typically falls between 30°C (86°F) and 225°C (437°F) at atmospheric pressure.
Factors Affecting the Boiling Point of Gasoline
-
Hydrocarbon Chain Length: The boiling point of gasoline is directly related to the length of the hydrocarbon chains. Shorter-chain alkanes, such as butane (C4H10) and pentane (C5H12), have lower boiling points compared to longer-chain alkanes, such as octane (C8H18) and decane (C10H22).
-
Degree of Branching: Branched-chain hydrocarbons generally have lower boiling points than their linear counterparts. For example, 2,2,4-trimethylpentane (isooctane) has a lower boiling point than n-octane.
-
Aromatic Compounds: Aromatic compounds, such as benzene, toluene, and xylenes, have higher boiling points compared to aliphatic hydrocarbons of similar molecular weight.
-
Pressure: The boiling point of gasoline is also influenced by the surrounding pressure. As the pressure increases, the boiling point of gasoline increases, and vice versa. This relationship is described by the Clausius-Clapeyron equation.
Atmospheric Distillation of Gasoline
The ASTM D86 method is the referee method for the certification of atmospheric distillation of light and middle distillate fuels, including gasoline. This method provides a standardized procedure for determining the boiling point distribution of gasoline.
The key parameters and findings from the ASTM D86 method include:
- Boiling Range: The boiling range of gasoline typically falls between 20°C (68°F) and 400°C (752°F) at atmospheric pressure.
- Car-Starting and Vapor-Lock Indexes: The front end of the distillation curve, which represents the lighter, more volatile components, affects the car-starting and vapor-lock indexes.
- Warm-Up Index: The middle and back end of the distillation curve, which represent the heavier, less volatile components, affect the warm-up index.
Simulated Distillation of Gasoline
Simulated distillation using gas chromatography is another method for determining the boiling point distribution of gasoline. This approach provides a more detailed and comprehensive analysis of the boiling point characteristics.
ASTM D7169: Boiling Point Distribution of Crude Oils and Residues
ASTM D7169 is a test method that covers the determination of the boiling point distribution and cut point intervals of crude oils and residues by using high-temperature gas chromatography. This method extends the applicability of simulated distillation to samples that do not elute completely from the chromatographic system, allowing for the determination of the boiling point distribution through a temperature of 720°C.
ASTM D7096: Boiling Range Distribution of Gasoline and Liquid Gasoline Blending Components
ASTM D7096 is a test method that covers the determination of the boiling range distribution of gasoline and liquid gasoline blending components by wide-bore capillary gas chromatography. This method is designed to measure the entire boiling range of gasoline and gasoline components, including those with high or low vapor pressure. It has been validated for gasoline containing ethanol and can estimate the concentration of n-pentane and lighter saturated hydrocarbons in gasoline.
The precision of ASTM D7096 has been evaluated through interlaboratory testing, and the repeatability and reproducibility of the test method have been found to exceed the values shown in Tables 7 and 8 for boiling point distribution and estimated gas concentrations in all but one case in twenty.
Practical Applications of Gasoline Boiling Point Data
The boiling point distribution of gasoline is crucial for various aspects of refinery operation and product quality control.
Refinery Operation
The boiling point distribution of gasoline provides important information for refinery operation, including:
- Potential Mass Percent Yield of Products: The boiling point distribution helps determine the potential mass percent yield of various refinery products, such as light ends, gasoline, kerosene, and diesel fuel.
- Amount of Residue Produced: The boiling point distribution also helps estimate the amount of residue produced during the refining process, which is essential for determining the economics of the refining operation.
Product Quality Control
The boiling point distribution of gasoline is also essential for product quality control, as it affects various performance characteristics of the fuel, such as:
- Car-Starting and Vapor-Lock Indexes: The front end of the distillation curve, which represents the lighter, more volatile components, affects the car-starting and vapor-lock indexes.
- Warm-Up Index: The middle and back end of the distillation curve, which represent the heavier, less volatile components, affect the warm-up index.
By understanding the boiling point distribution of gasoline, refiners can optimize their processes and ensure that the final product meets the necessary quality specifications for various end-use applications.
Conclusion
The boiling point of gasoline is a critical quality specification that is determined through various methods, including atmospheric distillation and simulated distillation using gas chromatography. The boiling point distribution of gasoline provides essential information for refinery operation and product quality control, allowing for the optimization of the refining process and the production of high-quality gasoline products.
References:
1. Lab Report: Initial Boiling Point
2. Comparative Online Distillation & Sulfur
3. EPA-R09-OAR-2022-0416-0048
4. ASTM D7096-10
5. ASTM D7169-20e01

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.