The boiling point of diethyl ether, a common organic solvent, is a crucial property that has significant implications in various scientific and industrial applications. This comprehensive guide delves into the intricacies of the boiling point of diethyl ether, providing a wealth of technical details and practical insights for science students and enthusiasts.
Understanding the Boiling Point of Diethyl Ether
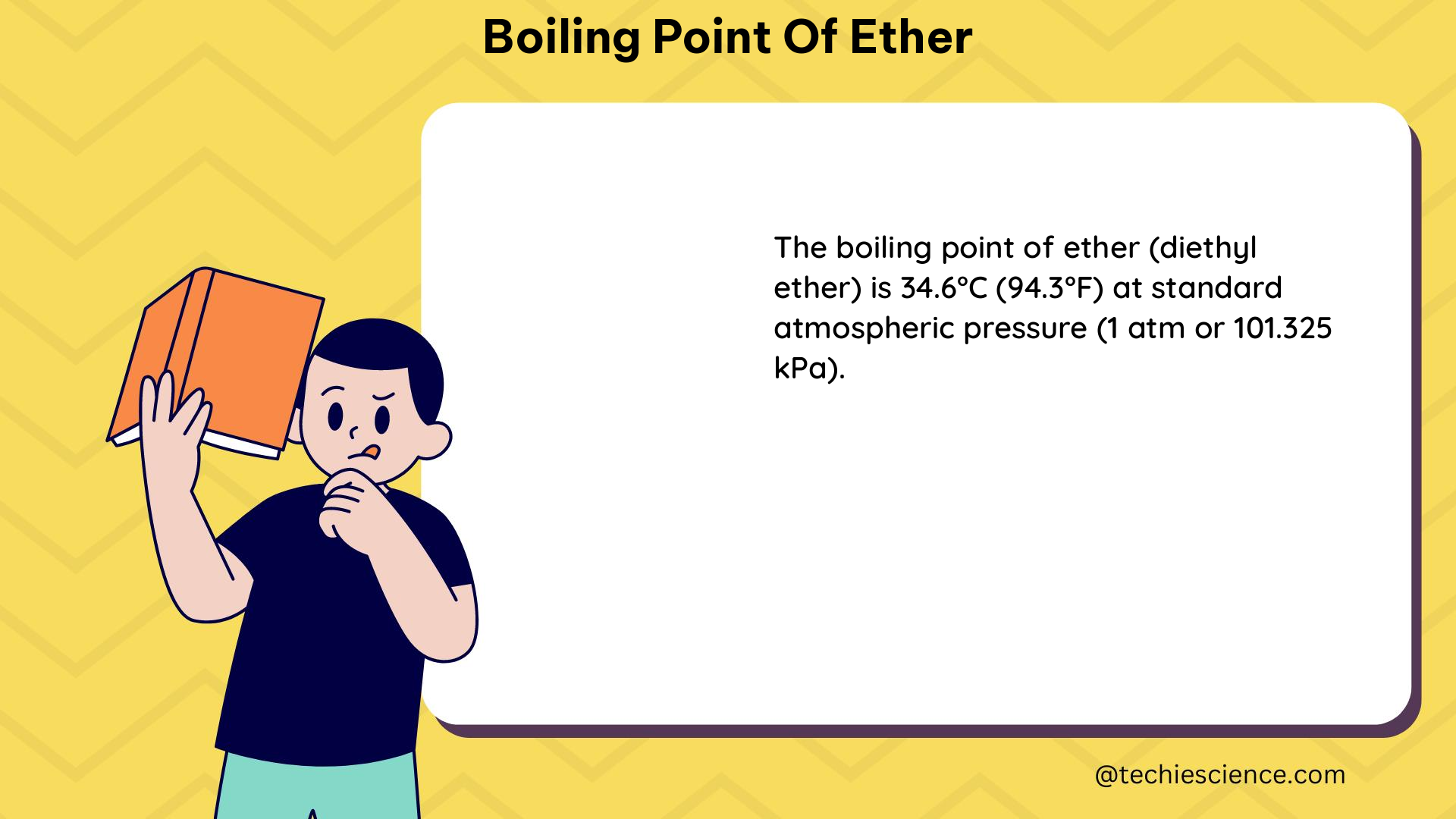
The boiling point of diethyl ether, also known as ethyl ether, is 34.5 degrees Celsius (94.3 degrees Fahrenheit) at a standard atmospheric pressure of 760 mmHg (millimeters of mercury). This value represents the temperature at which the vapor pressure of the liquid equals the surrounding atmospheric pressure, causing the liquid to transition into a gaseous state.
Molecular Structure and Intermolecular Forces
The relatively low boiling point of diethyl ether can be attributed to its molecular structure. The molecule consists of two ethyl groups (CH3CH2) connected by an oxygen atom. This arrangement results in weak intermolecular forces, such as van der Waals interactions, between the ether molecules. These weak forces allow the molecules to easily escape the liquid phase and transition into the gas phase, leading to the observed low boiling point.
Vapor Pressure and Volatility
In addition to the standard boiling point, the vapor pressure of diethyl ether is also an important property to consider. At a temperature of 25 degrees Celsius, the vapor pressure of diethyl ether is 541 mmHg. Vapor pressure is a measure of a substance’s volatility, and it is inversely related to the boiling point. A higher vapor pressure, as observed with diethyl ether, indicates a lower boiling point, as the molecules can more easily transition into the gas phase.
Theoretical Calculations of Boiling Point
Researchers have employed advanced computational methods to estimate the boiling point of diethyl ether. One such approach is the use of density functional theory (DFT) calculations with polarized continuum model (PCM) solvent corrections.
Density Functional Theory (DFT) Calculations
In a study published in the Journal of Chemical Physics, researchers used DFT calculations with PCM solvent corrections to estimate the boiling point of diethyl ether. The calculated boiling point was found to be 34.94 degrees Celsius (94.89 degrees Fahrenheit), which is in close agreement with the experimentally determined value.
The DFT calculations take into account the structural formula and electronic interactions between the diethyl ether molecules, allowing for a more accurate prediction of the boiling point. The PCM solvent corrections further refine the calculations by accounting for the effects of the surrounding environment on the ether molecules.
Practical Applications and Considerations
The boiling point of diethyl ether has significant implications in various scientific and industrial applications, including:
-
Solvent Applications: Diethyl ether is commonly used as a solvent in organic synthesis, extraction processes, and other laboratory procedures. The low boiling point of diethyl ether makes it a suitable choice for applications where a volatile solvent is required.
-
Fuel Applications: Diethyl ether has been explored as a potential fuel additive or alternative fuel due to its high energy density and low boiling point. The volatility of diethyl ether can be advantageous in certain engine applications.
-
Anesthetic Applications: Historically, diethyl ether has been used as a general anesthetic due to its ability to induce unconsciousness. The low boiling point and rapid evaporation of diethyl ether contribute to its anesthetic properties.
-
Safety Considerations: The low boiling point and high volatility of diethyl ether require careful handling and storage to mitigate the risk of fire and explosion. Proper safety protocols and ventilation are essential when working with diethyl ether.
Conclusion
The boiling point of diethyl ether is a fundamental property that has significant implications in various scientific and industrial applications. This comprehensive guide has explored the technical details, theoretical calculations, and practical considerations surrounding the boiling point of this important organic compound.
By understanding the factors that influence the boiling point, such as molecular structure and intermolecular forces, as well as the computational methods used to estimate it, science students and enthusiasts can gain a deeper appreciation for the complexities and applications of this essential property.
References:
- Chan, P. Y., Tong, C. M., & Durrant, M. C. (2011). Estimation of boiling points using density functional theory with polarized continuum model solvent corrections. Journal of Chemical Physics, 135(9), 094104.
- Diethyl Ether | (C2H5)2O | CID 3283 – PubChem. (n.d.). Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Diethyl-Ether
- Diethyl ether | C4H10O – ChemSpider. (n.d.). Retrieved from http://www.chemspider.com/Chemical-Structure.3168.html

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.