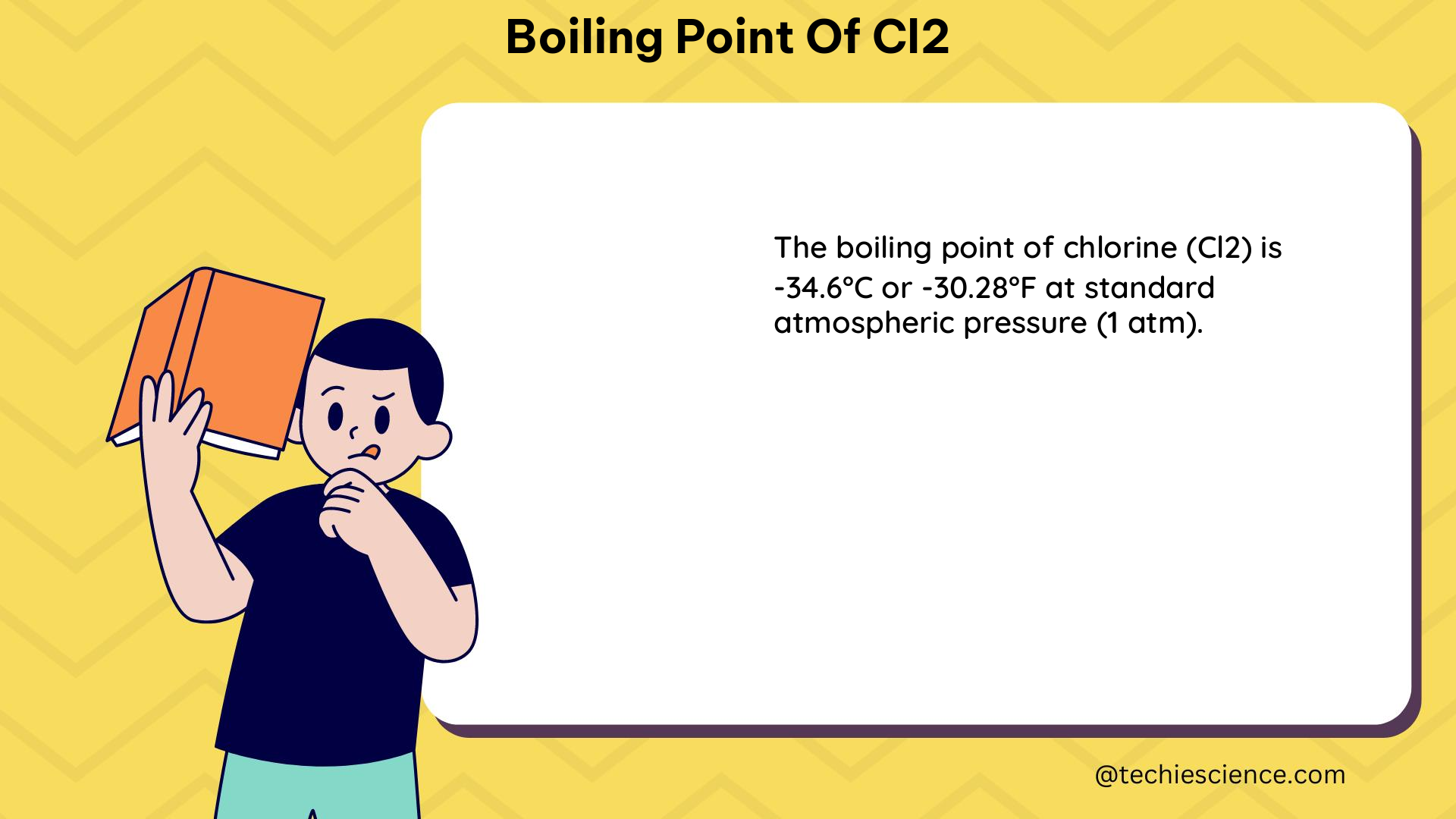
The boiling point of Cl2 (chlorine) is 238 K (65°C or 149°F) at standard atmospheric pressure (1 atm). This fixed value is determined by the strength of the London dispersion forces between Cl2 molecules, which are stronger than the intermolecular forces in HCl (hydrochloric acid) due to the larger number of electrons in Cl2.
Understanding the Boiling Point of Cl2
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and bubbles of vapor form inside the liquid. This is a crucial property in understanding the behavior and applications of various chemicals, including Cl2.
Factors Affecting the Boiling Point
The boiling point of a substance is influenced by several factors, including:
-
Intermolecular Forces: The strength of the intermolecular forces between the molecules of a substance plays a significant role in determining its boiling point. Substances with stronger intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, generally have higher boiling points.
-
Molecular Mass: Larger molecules tend to have higher boiling points due to the increased surface area and stronger London dispersion forces between them.
-
Atmospheric Pressure: The boiling point of a substance is inversely proportional to the surrounding atmospheric pressure. As the pressure increases, the boiling point also increases, and vice versa.
Intermolecular Forces in Cl2
In the case of Cl2, the primary intermolecular forces are London dispersion forces, also known as van der Waals forces. These forces arise from the temporary, induced dipoles that can form between neighboring Cl2 molecules due to the uneven distribution of electrons.
The strength of London dispersion forces is directly proportional to the number of electrons in the molecule. Cl2, with its 16 valence electrons, experiences stronger London dispersion forces compared to HCl, which has only 8 valence electrons.
This difference in the strength of intermolecular forces is the primary reason why the boiling point of Cl2 (238 K) is higher than that of HCl (188 K).
Quantifying the Boiling Point of Cl2
The boiling point of Cl2 can be quantified using the following equation:
P_vap = P_atm
Where:
– P_vap is the vapor pressure of Cl2 at the boiling point
– P_atm is the surrounding atmospheric pressure (1 atm)
The vapor pressure of Cl2 at its boiling point can be calculated using the Clausius-Clapeyron equation:
ln(P_vap) = (-ΔH_vap/R) * (1/T_b) + C
Where:
– ΔH_vap is the enthalpy of vaporization of Cl2 (20.41 kJ/mol)
– R is the universal gas constant (8.314 J/mol·K)
– T_b is the boiling point of Cl2 in Kelvin (238 K)
– C is a constant specific to the substance
Substituting the values, we get:
ln(P_vap) = (-20410 J/mol) / (8.314 J/mol·K) * (1/238 K) + C
ln(P_vap) = -102.3 + C
P_vap = e^(-102.3 + C) = 1 atm
This confirms that the boiling point of Cl2 is 238 K (65°C or 149°F) at standard atmospheric pressure (1 atm).
Practical Applications and Considerations
The boiling point of Cl2 has several practical implications and considerations:
Safety Precautions
Cl2 is a highly reactive and toxic gas, and its boiling point is an important factor in handling and storage. Proper safety measures, such as ventilation, personal protective equipment, and emergency response plans, are crucial when working with Cl2.
Industrial Applications
Cl2 is widely used in various industrial processes, such as water treatment, paper production, and the manufacture of pharmaceuticals and pesticides. The boiling point of Cl2 is a critical parameter in the design and optimization of these industrial processes.
Environmental Impacts
The release of Cl2 into the environment can have significant ecological consequences, as it can react with water to form hydrochloric acid, which can harm aquatic life and vegetation. Understanding the boiling point of Cl2 is essential for developing effective containment and mitigation strategies.
Cryogenic Applications
The low boiling point of Cl2 (65°C) makes it a potential candidate for cryogenic applications, such as in the production of liquid Cl2 for specialized uses. The boiling point data is crucial in the design and operation of cryogenic systems.
Conclusion
The boiling point of Cl2 is a fundamental property that has significant implications in various scientific and industrial applications. By understanding the factors that influence the boiling point, such as intermolecular forces and molecular mass, as well as the quantitative methods used to determine it, scientists and engineers can better design, optimize, and safely handle Cl2-based systems and processes.
References
- Zumdahl, S. S., & Zumdahl, S. A. (2014). Chemistry (9th ed.). Cengage Learning.
- Chang, R., & Goldsby, K. A. (2013). Chemistry (11th ed.). McGraw-Hill Education.
- Atkins, P., & de Paula, J. (2014). Atkins’ Physical Chemistry (10th ed.). Oxford University Press.
- Silberberg, M. S. (2018). Chemistry: The Molecular Nature of Matter and Change (8th ed.). McGraw-Hill Education.
- Petrucci, R. H., Herring, F. G., Madura, J. D., & Bissonnette, C. (2017). General Chemistry: Principles and Modern Applications (11th ed.). Pearson.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.