The boiling point of CH3CH2OH, also known as ethanol or ethyl alcohol, is a crucial physical property that plays a vital role in various scientific and industrial applications. This comprehensive guide will delve into the intricacies of the boiling point of CH3CH2OH, providing science students with a detailed understanding of this fundamental concept.
Understanding the Boiling Point of CH3CH2OH
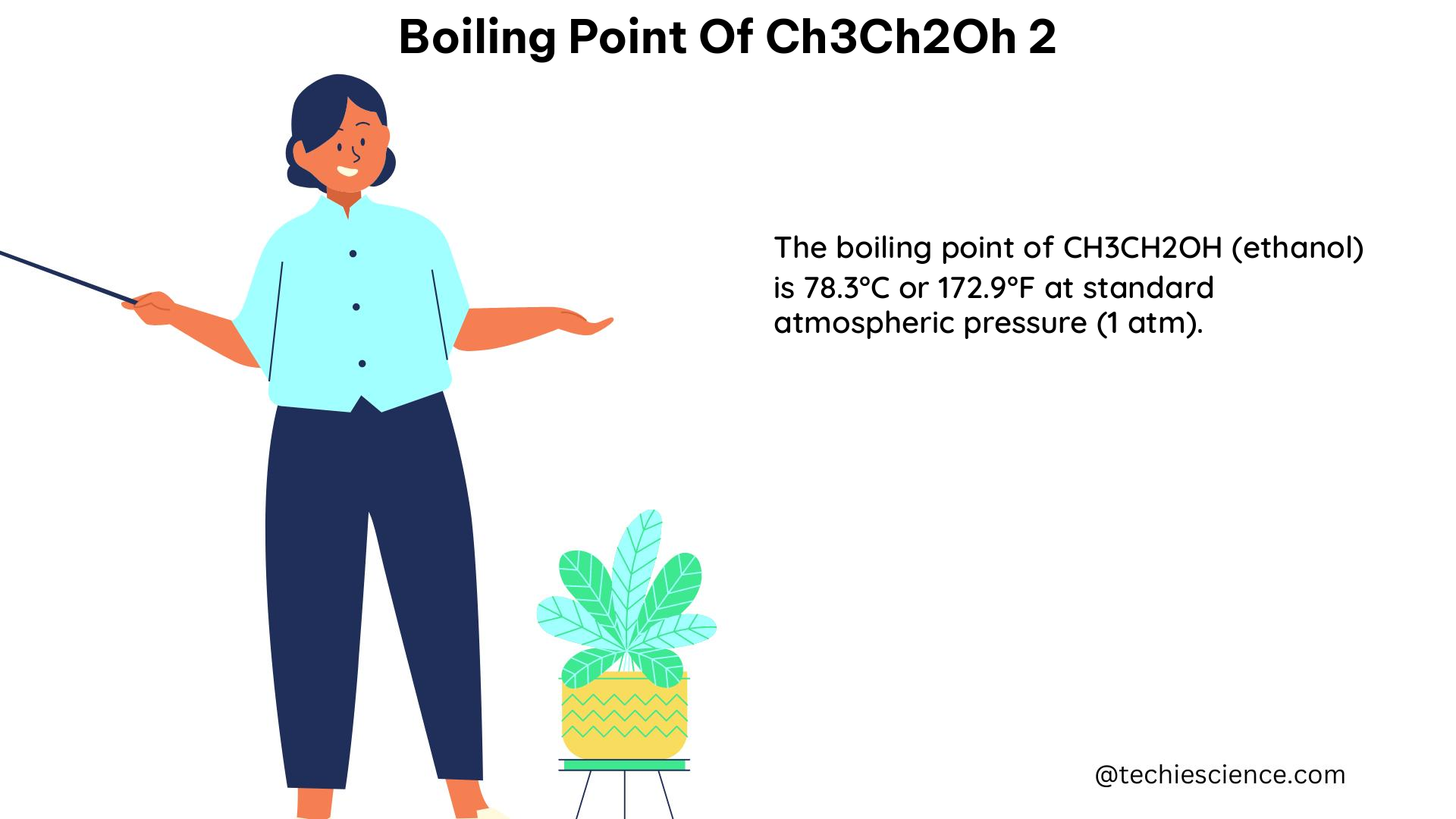
The boiling point of CH3CH2OH is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and bubbles of vapor form inside the liquid. At this point, the liquid changes into a gas. The boiling point of CH3CH2OH is 78.37°C (173.1°F) at standard atmospheric pressure (1 atm or 101.325 kPa).
The Clausius-Clapeyron Equation
The relationship between the boiling point and the vapor pressure of a substance can be described by the Clausius-Clapeyron equation:
ln(P2/P1) = (ΔHvap/R) * (1/T1 - 1/T2)
Where:
– P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively.
– ΔHvap is the enthalpy of vaporization of the substance.
– R is the universal gas constant (8.314 J/mol·K).
This equation can be used to calculate the boiling point of CH3CH2OH at different pressures or to determine the enthalpy of vaporization from experimental data.
Factors Affecting the Boiling Point of CH3CH2OH
The boiling point of CH3CH2OH can be influenced by several factors, including:
-
Intermolecular Forces: The strength of the intermolecular forces, such as hydrogen bonding, between CH3CH2OH molecules can affect the boiling point. Stronger intermolecular forces require more energy to overcome, resulting in a higher boiling point.
-
Molecular Structure: The shape and size of the CH3CH2OH molecule can also influence the boiling point. Larger molecules generally have higher boiling points due to increased van der Waals forces.
-
Pressure: As mentioned earlier, the boiling point of CH3CH2OH is directly related to the surrounding pressure. Increasing the pressure will raise the boiling point, while decreasing the pressure will lower it.
-
Impurities: The presence of impurities in the CH3CH2OH sample can affect the boiling point. Impurities can disrupt the intermolecular forces, leading to a change in the boiling point.
Boiling Point Elevation and Azeotropes
When CH3CH2OH is mixed with other substances, such as water, the boiling point of the mixture can be different from the boiling point of pure CH3CH2OH. This phenomenon is known as boiling point elevation or depression, depending on the nature of the solute.
In the case of a CH3CH2OH-water mixture, the boiling point of the mixture is lower than the boiling point of pure water due to the formation of an azeotrope. An azeotrope is a constant boiling point mixture, where the composition of the vapor phase is the same as the liquid phase. This is because the presence of CH3CH2OH disrupts the hydrogen bonding network in water, leading to a decrease in the boiling point.
Applications of the Boiling Point of CH3CH2OH
The boiling point of CH3CH2OH has numerous applications in various scientific and industrial fields, including:
-
Distillation and Purification: The difference in boiling points between CH3CH2OH and other compounds allows for the separation and purification of CH3CH2OH through distillation processes. This is particularly important in the production of alcoholic beverages, biofuels, and pharmaceutical products.
-
Chemical Reactions and Synthesis: The boiling point of CH3CH2OH is a crucial parameter in chemical reactions and synthesis, as it determines the optimal temperature for the reaction to occur and the conditions for product separation.
-
Thermodynamic Studies: The boiling point of CH3CH2OH is a fundamental property used in thermodynamic studies, such as the calculation of enthalpy of vaporization, entropy changes, and the determination of phase diagrams.
-
Fuel and Energy Applications: CH3CH2OH is used as a fuel additive in gasoline and as a renewable biofuel. The boiling point of CH3CH2OH is an important factor in the design and optimization of engines and fuel systems.
-
Biological and Medical Applications: CH3CH2OH is used in various medical and biological applications, such as disinfectants, antiseptics, and as a solvent for pharmaceutical compounds. The boiling point of CH3CH2OH is a crucial parameter in these applications.
Numerical Examples and Calculations
To further illustrate the importance of the boiling point of CH3CH2OH, let’s consider some numerical examples and calculations:
-
Calculating the Vapor Pressure of CH3CH2OH at a Given Temperature:
Using the Clausius-Clapeyron equation, we can calculate the vapor pressure of CH3CH2OH at a specific temperature. For example, at 25°C, the vapor pressure of CH3CH2OH is approximately 5.95 kPa. -
Determining the Enthalpy of Vaporization of CH3CH2OH:
Rearranging the Clausius-Clapeyron equation, we can solve for the enthalpy of vaporization (ΔHvap) of CH3CH2OH. Given the boiling point of 78.37°C and the vapor pressure at 25°C, the enthalpy of vaporization is approximately 38.56 kJ/mol. -
Analyzing the Boiling Point Elevation in a CH3CH2OH-Water Mixture:
Suppose a mixture of 50% CH3CH2OH and 50% water is heated. The boiling point of the mixture will be lower than the boiling point of pure water (100°C) due to the formation of an azeotrope. The exact boiling point of the mixture can be calculated using the Raoult’s law and the vapor-liquid equilibrium relationships.
These examples demonstrate the versatility and importance of understanding the boiling point of CH3CH2OH in various scientific and engineering applications.
Conclusion
The boiling point of CH3CH2OH is a fundamental physical property that plays a crucial role in numerous scientific and industrial applications. By understanding the factors that influence the boiling point, the underlying principles, and the practical applications, science students can develop a comprehensive understanding of this essential concept. This guide has provided a detailed exploration of the boiling point of CH3CH2OH, equipping students with the knowledge and tools necessary to navigate the complexities of this topic.
Reference:
- Atkins, P., & de Paula, J. (2014). Atkins’ Physical Chemistry (10th ed.). Oxford University Press.
- Rouessac, F., & Rouessac, A. (2013). Chemical Analysis: Modern Instrumentation Methods and Techniques (2nd ed.). Wiley.
- Silbey, R. J., Alberty, R. A., & Bawendi, M. G. (2005). Physical Chemistry (4th ed.). Wiley.
- Levine, I. N. (2009). Physical Chemistry (6th ed.). McGraw-Hill.
- Engel, T., & Reid, P. (2013). Physical Chemistry (3rd ed.). Pearson.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.