The boiling point of butane, a widely used fuel and refrigerant, is a crucial property that determines its behavior and applications. This comprehensive guide delves into the technical details, physics principles, and practical implications of the boiling point of butane, providing a valuable resource for science students and enthusiasts.
Understanding the Boiling Point of Butane
The boiling point of a substance, in this case, butane, is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and bubbles of vapor form inside the liquid. This transition from liquid to gas phase occurs at a specific temperature, known as the normal boiling point, under standard atmospheric pressure (1 atm or 101.3 kPa).
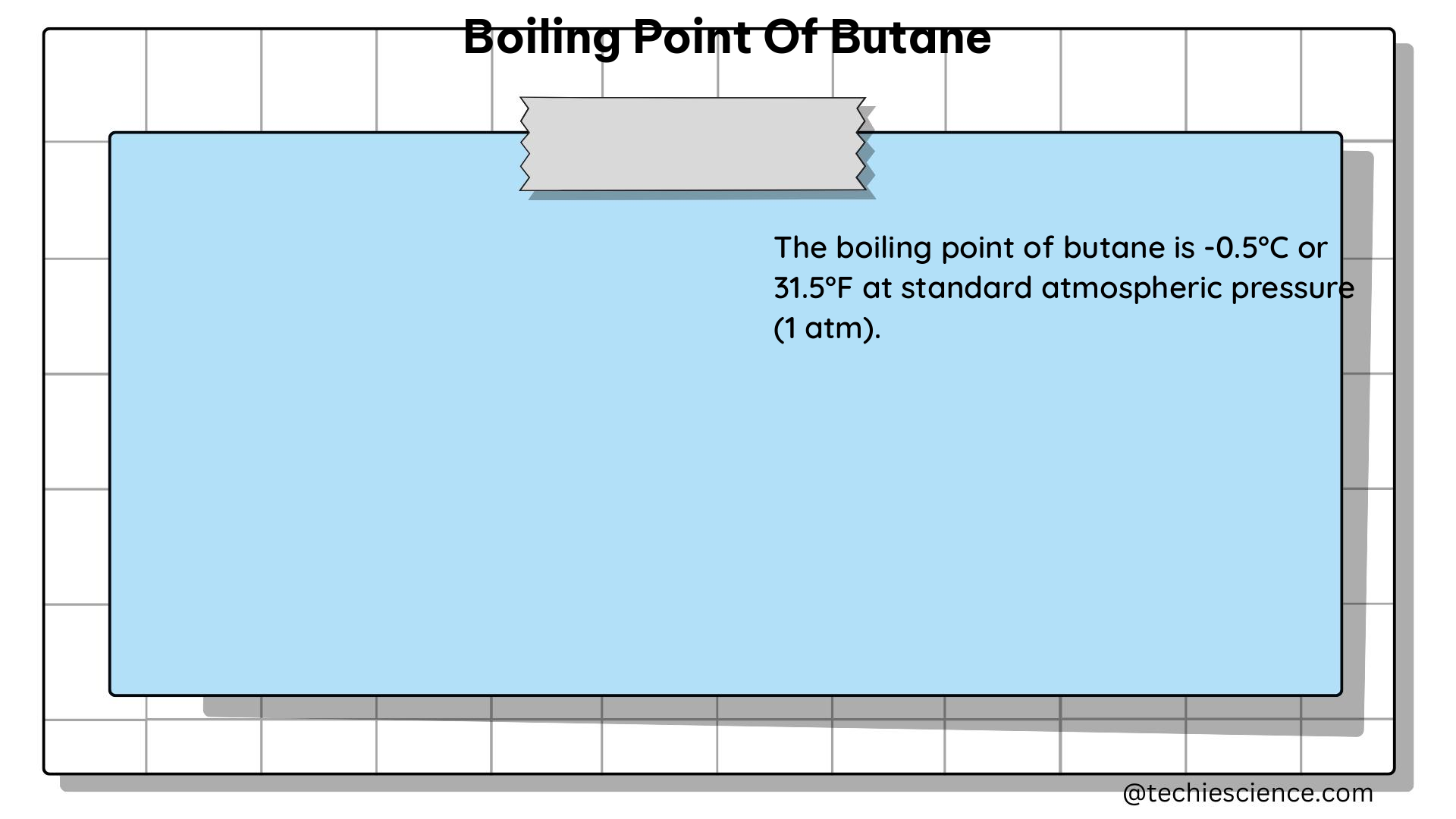
The boiling point of butane is consistently reported to be around -0.5°C (31.1°F) under standard conditions. This value is derived from numerous experiments and data compilations, ensuring its accuracy and reliability.
Factors Affecting the Boiling Point of Butane
The boiling point of butane is influenced by several factors, including:
-
Molecular Structure: The boiling point of butane is primarily determined by its molecular structure, specifically the intermolecular forces between the butane molecules. Butane, with the chemical formula C₄H₁₀, has a relatively simple linear structure, which results in weaker intermolecular van der Waals forces compared to larger or more complex hydrocarbon molecules.
-
Pressure: The boiling point of a substance is inversely proportional to the surrounding pressure. As the pressure increases, the boiling point also increases, and vice versa. This relationship is described by the Clausius-Clapeyron equation:
ln(P₂/P₁) = (ΔHvap/R) * (1/T₁ - 1/T₂)
where P₁ and P₂ are the vapor pressures at temperatures T₁ and T₂, respectively, ΔHvap is the enthalpy of vaporization, and R is the universal gas constant.
-
Impurities: The presence of impurities in the butane can affect its boiling point. Impurities can interact with the butane molecules, altering the intermolecular forces and, consequently, the boiling point.
-
Isotopic Composition: The boiling point of butane can also be influenced by its isotopic composition. Variations in the relative abundance of different isotopes of carbon and hydrogen can result in slight differences in the boiling point.
Practical Applications of Butane’s Boiling Point
The boiling point of butane has several practical applications:
-
Fuel and Propellant: Butane’s low boiling point makes it an ideal fuel for various applications, such as lighters, portable stoves, and camping equipment. The low boiling point allows butane to vaporize easily, providing a reliable and efficient fuel source.
-
Refrigerant: Butane’s boiling point of -0.5°C (31.1°F) makes it a suitable refrigerant for small-scale refrigeration systems, such as those found in portable coolers or mini-fridges.
-
Aerosol Propellant: The low boiling point of butane allows it to be used as a propellant in aerosol cans, where the pressurized gas is used to expel the contents of the can.
-
Chemical Synthesis: The boiling point of butane is an important parameter in various chemical synthesis processes, where the controlled vaporization and condensation of butane are crucial.
Numerical Examples and Calculations
-
Calculating Vapor Pressure: Using the Clausius-Clapeyron equation, we can calculate the vapor pressure of butane at a given temperature. For example, at 20°C, the vapor pressure of butane is approximately 1.92 bar.
-
Determining Boiling Point at Different Pressures: Applying the Clausius-Clapeyron equation, we can find the boiling point of butane at different pressures. For instance, at a pressure of 2 atm, the boiling point of butane would be approximately 3.8°C.
-
Estimating Enthalpy of Vaporization: Rearranging the Clausius-Clapeyron equation, we can estimate the enthalpy of vaporization (ΔHvap) of butane. Using the known boiling point and vapor pressure data, the enthalpy of vaporization is calculated to be around 23.2 kJ/mol.
-
Comparing Boiling Points of Isomers: The boiling point of butane (n-butane) is -0.5°C, while the boiling point of its isomer, isobutane, is -11.7°C. This difference in boiling points is due to the variations in their molecular structures and intermolecular forces.
Graphical Representation and Data Visualization
To better understand the relationship between the boiling point of butane and various factors, we can utilize graphical representations and data visualization techniques:
-
Pressure-Temperature Phase Diagram: A phase diagram can be used to illustrate the phase transitions of butane as a function of pressure and temperature. This diagram would show the liquid-gas equilibrium line, indicating the boiling point at different pressures.
-
Vapor Pressure Curve: A plot of the vapor pressure of butane as a function of temperature can provide insights into the relationship between these two variables, as described by the Clausius-Clapeyron equation.
-
Comparison of Boiling Points: A bar graph or a table can be used to compare the boiling points of butane and its isomers, highlighting the structural differences and their impact on the boiling point.
Conclusion
The boiling point of butane is a crucial property that has significant implications in various scientific and industrial applications. This comprehensive guide has explored the technical details, physics principles, and practical applications of the boiling point of butane, providing a valuable resource for science students and enthusiasts. By understanding the factors that influence the boiling point and the associated calculations and visualizations, we can gain a deeper appreciation for the behavior and versatility of this important substance.
References
- NIST Chemistry WebBook: https://webbook.nist.gov/cgi/cbook.cgi?ID=C106978&Type=TBOIL
- Thales Group: https://info.thalesgroup.com/en/sites/default/files/2019-02/Butane-Liquefied-Petroleum-Gas-LPG-Data-Sheet-DS-011-E-EN.pdf
- TCEQ Texas: https://www.tceq.texas.gov/downloads/toxicology/dsd/final/butanes.pdf
- Clausius-Clapeyron Equation: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Liquids/Vapor_Pressure/The_Clausius-Clapeyron_Equation

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.