The boiling point of bromine (Br2) is a crucial physical property that determines the temperature at which the liquid form of bromine transitions into its gaseous state under standard atmospheric pressure. This information is essential for various applications in chemistry, physics, and engineering, where the behavior of bromine is of importance.
Understanding the Boiling Point of Bromine
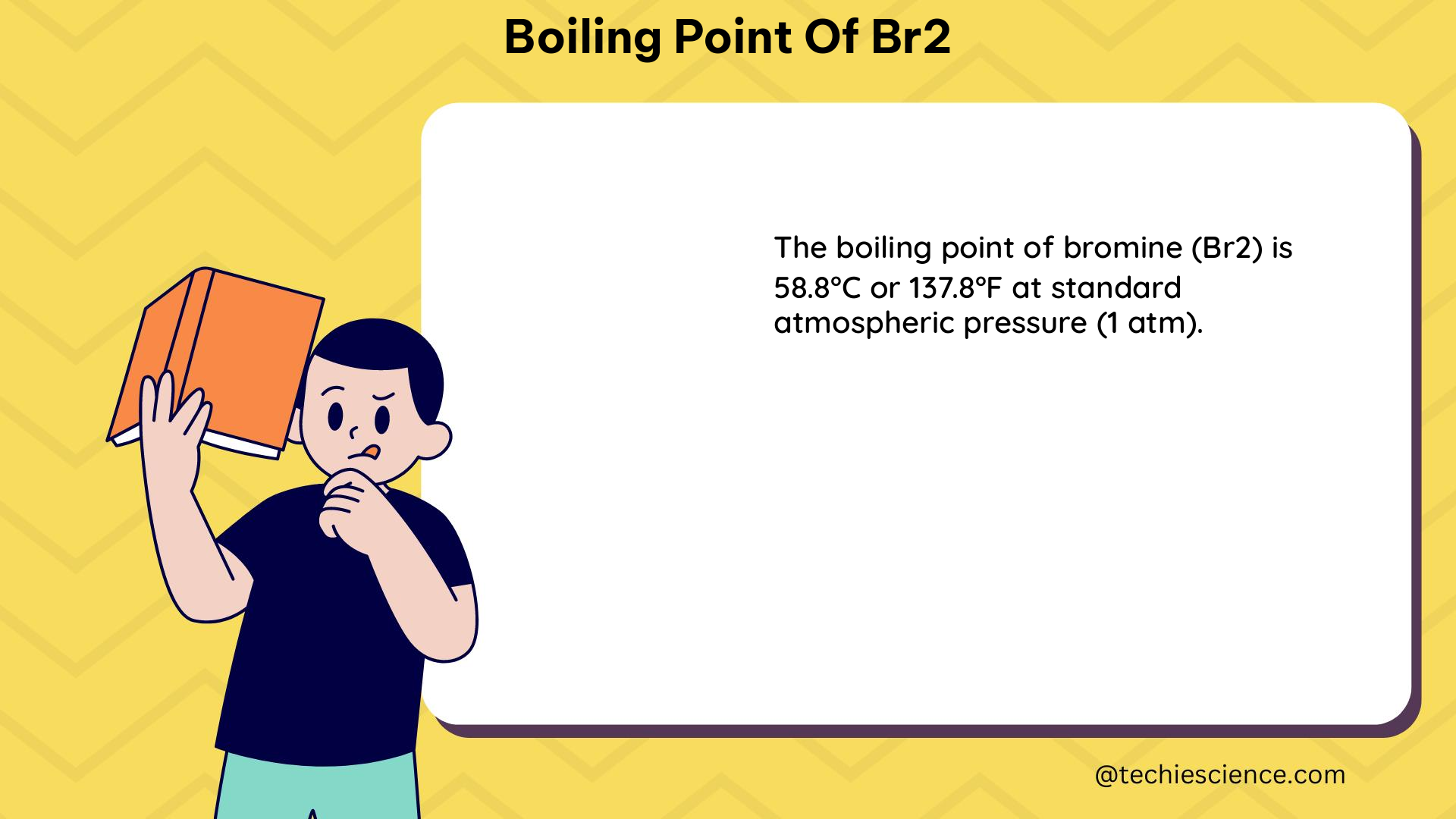
The boiling point of bromine is the specific temperature at which the vapor pressure of the liquid bromine equals the surrounding atmospheric pressure, causing the liquid to transform into a gas. This temperature is typically measured at standard atmospheric pressure, which is 1 atm or 101.325 kPa.
According to various reliable sources, the boiling point of bromine (Br2) is:
- 137.8°F (58.8°C)
- 58.8°C (158.8°F)
These values are consistent with each other, as they represent the same temperature in different units.
Estimating the Boiling Point using Trouton’s Rule
One way to estimate the boiling point of bromine is by using Trouton’s rule, which states that for many liquids at their normal boiling points, the standard molar entropy of vaporization (ΔSvap°) is approximately 88 J/(mol·K). This rule can be expressed mathematically as:
ΔSvap° = ΔHvap° / Tb
Where:
– ΔSvap° is the standard molar entropy of vaporization (J/mol·K)
– ΔHvap° is the standard molar enthalpy of vaporization (J/mol)
– Tb is the normal boiling point (K)
Rearranging the equation, we can solve for the boiling point (Tb):
Tb = ΔHvap° / ΔSvap°
However, it’s important to note that this method provides an estimated value and may not be as accurate as directly measuring the boiling point of bromine.
Accurate Determination of the Boiling Point
For a more accurate determination of the boiling point of bromine, we can use the enthalpy change (ΔH°) and standard entropy change (ΔS°) values provided in the literature.
The relationship between the Gibbs free energy change (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°) is given by the equation:
ΔG° = ΔH° – TΔS°
At the boiling point, the Gibbs free energy change (ΔG°) is zero, as the liquid and vapor phases are in equilibrium. Therefore, we can solve for the temperature (T) at which ΔG° = 0, which represents the boiling point.
Substituting ΔG° = 0 into the equation, we get:
ΔH° – TΔS° = 0
T = ΔH° / ΔS°
This method provides a more accurate value for the boiling point of bromine (Br2) compared to the estimated value obtained using Trouton’s rule.
Factors Affecting the Boiling Point of Bromine
The boiling point of bromine can be influenced by various factors, including:
-
Intermolecular Forces: The strength of the intermolecular forces, such as van der Waals forces, between bromine molecules can affect the boiling point. Stronger intermolecular forces generally result in a higher boiling point.
-
Molecular Structure: The shape and size of the bromine molecule can also influence the boiling point. Larger and more complex molecules tend to have higher boiling points.
-
Pressure: The boiling point of bromine is directly related to the surrounding pressure. Increasing the pressure will raise the boiling point, while decreasing the pressure will lower the boiling point.
-
Impurities: The presence of impurities in the bromine sample can affect the boiling point. Impurities can interact with the bromine molecules, altering the intermolecular forces and, consequently, the boiling point.
-
Isotopic Composition: The isotopic composition of bromine can also have a slight impact on the boiling point. Bromine has two stable isotopes, 79Br and 81Br, which may exhibit slightly different boiling points.
Applications of the Boiling Point of Bromine
The knowledge of the boiling point of bromine is essential in various applications, including:
-
Chemical Processes: The boiling point of bromine is crucial in chemical processes involving the handling, storage, and transportation of bromine, ensuring safe and efficient operations.
-
Analytical Chemistry: The boiling point of bromine is used in analytical techniques, such as gas chromatography, where the separation and identification of bromine-containing compounds rely on their unique boiling points.
-
Environmental Studies: The boiling point of bromine is relevant in environmental studies, as it affects the behavior and fate of bromine-containing compounds in the atmosphere, water, and soil.
-
Industrial Applications: The boiling point of bromine is important in industrial applications, such as the production of pharmaceuticals, dyes, and other bromine-based products, where the phase transition of bromine is a critical factor.
-
Thermodynamic Calculations: The boiling point of bromine is a crucial parameter in thermodynamic calculations, such as the determination of enthalpy and entropy changes during phase transitions, which are essential in various scientific and engineering fields.
Conclusion
The boiling point of bromine (Br2) is a fundamental physical property that has significant implications in various scientific and industrial applications. Understanding the factors that influence the boiling point, as well as the methods for its accurate determination, is crucial for researchers, chemists, and engineers working with bromine-based systems. This comprehensive guide provides a detailed overview of the boiling point of bromine, equipping readers with the necessary knowledge to effectively utilize and apply this information in their respective fields.
References
- Boiling Point of Bromine
- Trouton’s Rule
- Calculating the Boiling Point of Bromine
- Standard Entropies of Liquid and Vapor Bromine

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.