The boiling point of boron, a metalloid element with the atomic number 5, is a crucial physical property that has been extensively studied and documented. This comprehensive guide delves into the intricacies of boron’s boiling point, providing a wealth of technical details and quantifiable data to aid science students and enthusiasts in their understanding of this fascinating topic.
Understanding the Boiling Point of Elemental Boron
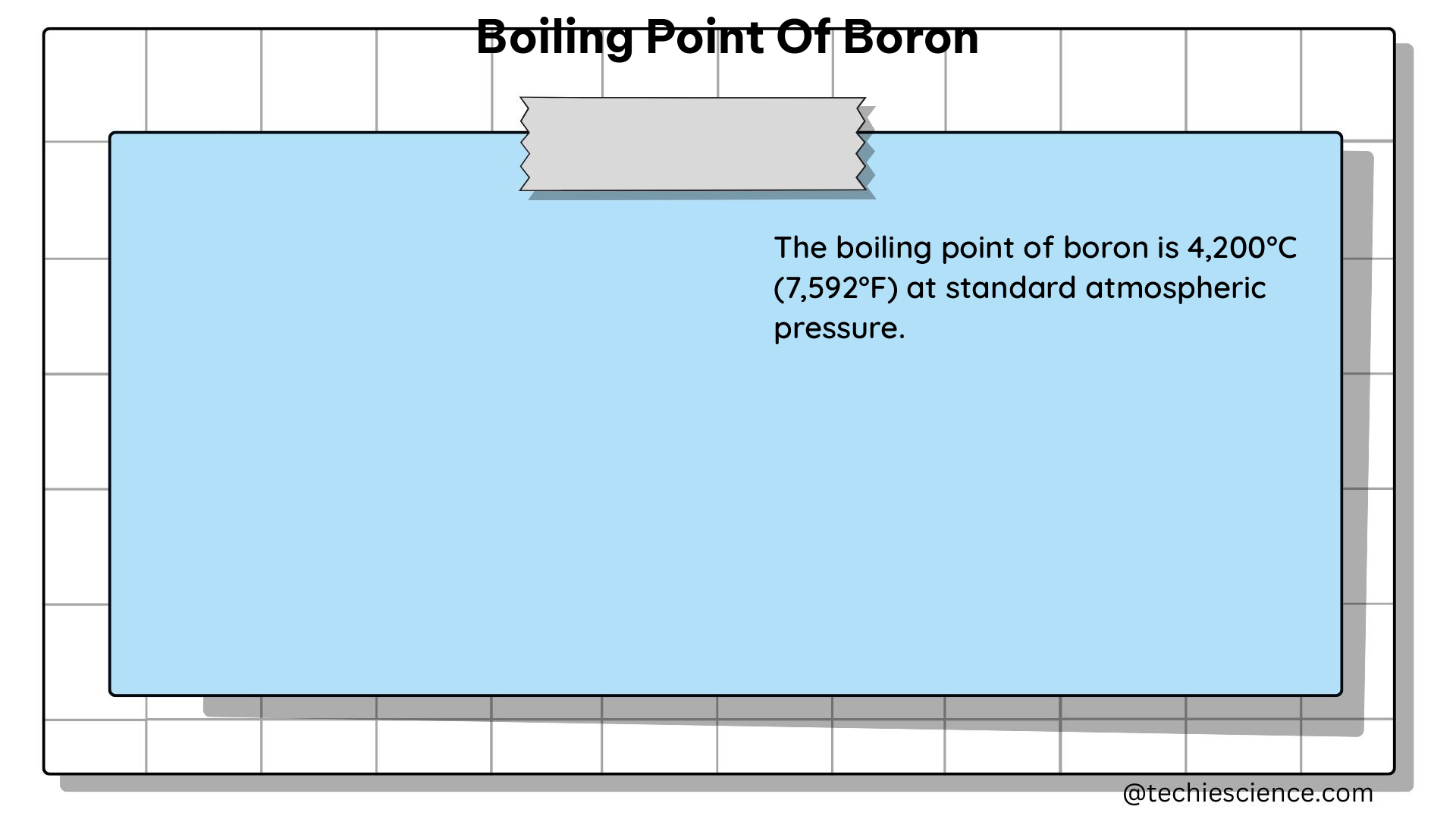
According to the ATSDR Toxicological Profile for Boron, the boiling point of elemental boron is 4,000 °C (7,232 °F) at atmospheric pressure. This value represents the temperature at which the vapor pressure of boron equals the surrounding pressure, causing the liquid to transition into a gaseous state.
It is important to note that this boiling point refers specifically to the pure, elemental form of boron, which is not commonly encountered in practical applications. Boron is more frequently found in the form of various compounds, each with its own unique boiling point characteristics.
Boiling Points of Boron Compounds
While the boiling point of elemental boron is exceptionally high, boron compounds often exhibit lower boiling points due to their more complex molecular structures. Let’s explore the boiling points of some common boron compounds:
Boric Acid (H3BO3)
The boiling point of boric acid (H3BO3) is around 150-155 °C (302-311 °F) at atmospheric pressure. This lower boiling point is a result of the compound’s molecular structure, which includes hydrogen and oxygen atoms in addition to the boron atom.
Boron Oxide (B2O3)
The boiling point of boron oxide (B2O3) is approximately 1,650 °C (2,982 °F) at atmospheric pressure. While still significantly lower than the boiling point of elemental boron, the boiling point of boron oxide is considerably higher than that of boric acid due to its different molecular composition.
Factors Affecting the Boiling Point of Boron
The boiling point of boron can be influenced by various factors, including the crystalline form of the element, pressure, temperature, and the presence of impurities. Understanding these factors is crucial for accurately measuring and interpreting the boiling point of boron.
Crystalline Form
The boiling point of boron can vary depending on the specific crystalline form of the element. For instance, the boiling point of amorphous boron is around 2,250 °C (4,082 °F), while the boiling point of crystalline boron can differ depending on the crystal structure.
Pressure and Temperature
The boiling point of boron, like any substance, is affected by changes in pressure and temperature. As pressure increases, the boiling point of boron also increases, following the Clausius-Clapeyron equation:
ln(P2/P1) = (ΔHvap/R) * (1/T1 - 1/T2)
where P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively, ΔHvap is the enthalpy of vaporization, and R is the universal gas constant.
Conversely, as temperature increases, the boiling point of boron decreases, as the vapor pressure of the element rises.
Impurities
The presence of impurities in boron can also affect its boiling point. Impurities can alter the chemical and physical properties of the element, potentially leading to changes in the boiling point. It is essential to specify the purity and composition of the boron sample when reporting its boiling point to ensure accurate and consistent measurements.
Practical Applications and Considerations
The high boiling point of boron has significant implications in various scientific and industrial applications. Understanding the boiling point of boron is crucial for processes such as:
-
Boron Doping in Semiconductor Manufacturing: Boron is commonly used as a dopant in the production of semiconductor devices, where its high boiling point is a key consideration in the fabrication process.
-
Boron Carbide (B4C) Production: Boron carbide, a ceramic material with exceptional hardness, is produced through the reaction of boron and carbon at high temperatures. The boiling point of boron is a critical factor in this process.
-
Boron Neutron Capture Therapy (BNCT): BNCT is a cancer treatment that utilizes the high neutron capture cross-section of boron-10 isotope. The boiling point of boron is an important parameter in the design and optimization of BNCT systems.
-
Boron-Based Rocket Propellants: Boron and its compounds are sometimes used as high-energy additives in solid and liquid rocket propellants, where the boiling point of boron is a crucial consideration in the formulation and performance of these propellants.
-
Boron Fiber Reinforced Composites: The high boiling point of boron enables the production of boron fibers, which are used to reinforce advanced composite materials with exceptional strength and stiffness.
In summary, the boiling point of boron is a well-defined physical property that varies depending on the form of the element and the measurement conditions. Understanding the factors that influence the boiling point of boron is crucial for various scientific and industrial applications, from semiconductor manufacturing to advanced materials development.
References
- Boron elemental and isotopic determination via the BF diatomic molecule by graphite furnace atomic absorption spectrometry, published in the Journal of Analytical Chemistry in 2024.
- Chem 180 set 2 Flashcards, published on Quizlet in 2024.
- Combustion of Boron Particles at Atmospheric Pressure, published by the Defense Technical Information Center in 1966.
- Combustion of boron particles at elevated pressures, published in the Journal of Applied Chemistry and Biotechnology in 1971.
- ATSDR Toxicological Profile for Boron, published by the Agency for Toxic Substances and Disease Registry in 2021.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.