Summary
Benzoic acid, a widely used organic compound, has a well-defined boiling point of 250 °C (482 °F; 523 K) at standard atmospheric pressure (760 mmHg or 1 atm). This physical property is crucial in understanding the phase transitions, synthesis, purification, and applications of benzoic acid. In this comprehensive guide, we will delve into the technical details, underlying principles, and practical implications of the boiling point of benzoic acid.
Understanding the Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and bubbles of vapor form inside the liquid. This transition from the liquid phase to the gas phase is a critical point in the phase diagram of a substance.
Clausius-Clapeyron Equation
The relationship between the boiling point and the vapor pressure of a substance can be described by the Clausius-Clapeyron equation:
ln(P2/P1) = (ΔHvap/R) * (1/T1 - 1/T2)
Where:
– P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively
– ΔHvap is the enthalpy of vaporization
– R is the universal gas constant
This equation allows us to predict the boiling point of a substance at different pressures, as well as the relationship between the boiling point and the vapor pressure.
Factors Affecting Boiling Point
The boiling point of a substance can be influenced by various factors, including:
-
Molecular Structure: The intermolecular forces, such as hydrogen bonding, dipole-dipole interactions, and van der Waals forces, can affect the boiling point. Substances with stronger intermolecular forces generally have higher boiling points.
-
Pressure: As per the Clausius-Clapeyron equation, the boiling point increases with increasing pressure and decreases with decreasing pressure.
-
Impurities: The presence of impurities in a substance can alter its boiling point. Impurities can either increase or decrease the boiling point, depending on their nature and concentration.
-
Solvent Effects: When a substance is dissolved in a solvent, the boiling point of the solution may differ from the pure substance due to the interactions between the solute and the solvent.
Boiling Point of Benzoic Acid
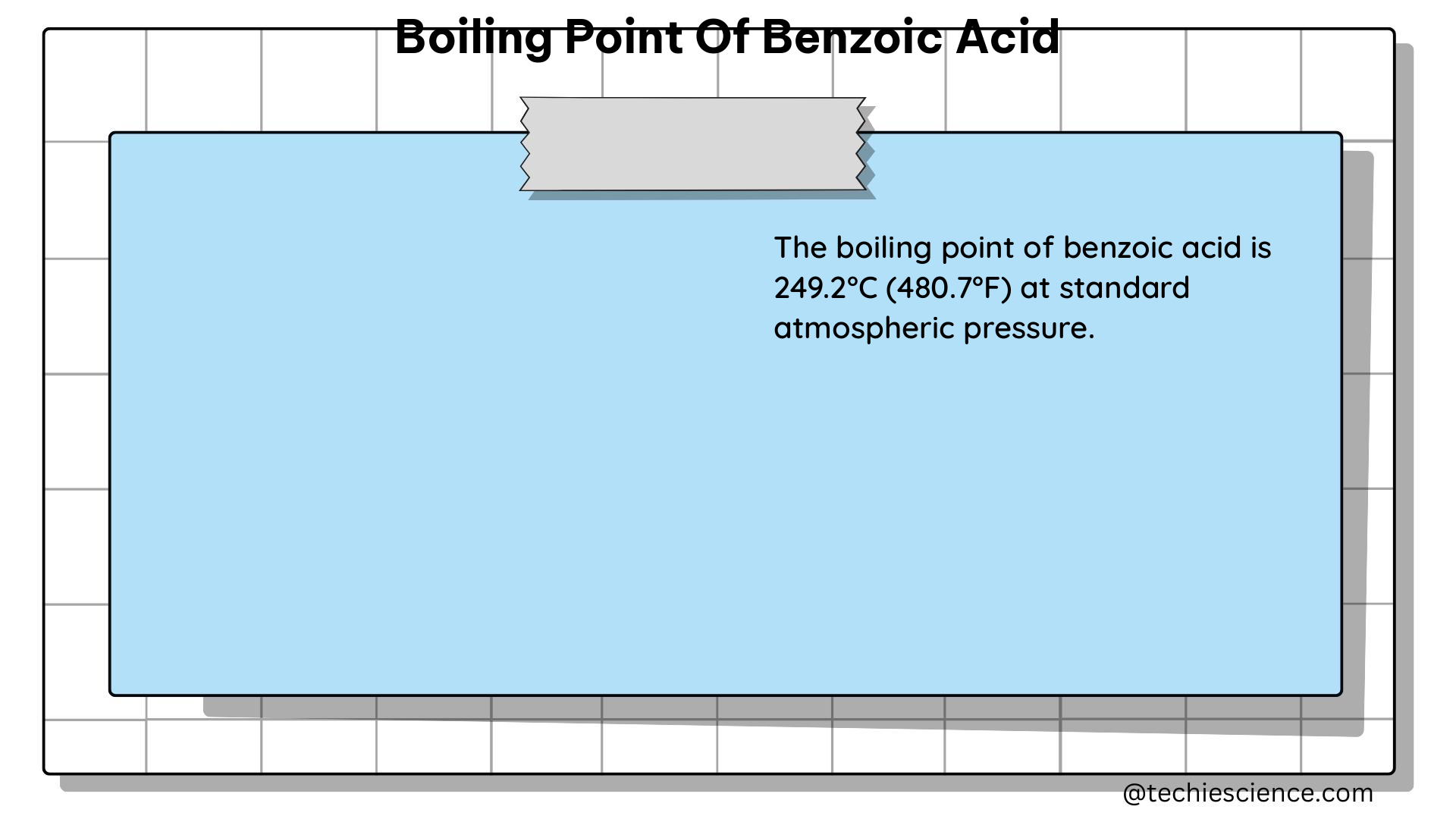
Benzoic acid, with the chemical formula C₆H₅COOH, has a boiling point of 250 °C (482 °F; 523 K) at standard atmospheric pressure (760 mmHg or 1 atm). This value is consistent across various sources and is a well-established physical property of benzoic acid.
Practical Implications of Boiling Point
The boiling point of benzoic acid has several practical implications in its synthesis, purification, and applications.
Synthesis and Purification
-
Recrystallization: The boiling point of the solvent used in the recrystallization process should be close to, but not equal to, the boiling point of benzoic acid. This ensures efficient dissolution and crystallization of the compound.
-
Distillation: The boiling point of benzoic acid is a crucial factor in the design and optimization of distillation columns and other separation techniques used in the purification of benzoic acid.
-
Sublimation: Benzoic acid can also be purified by sublimation, where the compound is heated to its boiling point, and the vapor is condensed to obtain the pure substance.
Applications
-
Food Preservative: Benzoic acid is commonly used as a food preservative due to its antimicrobial properties. The boiling point is an important consideration in the formulation and storage of benzoic acid-based preservatives.
-
Pharmaceutical Industry: Benzoic acid and its derivatives are used in the pharmaceutical industry as intermediates in the synthesis of various drugs. The boiling point is a critical parameter in the purification and processing of these compounds.
-
Chemical Industry: Benzoic acid is an important building block in the synthesis of various organic compounds, such as dyes, plasticizers, and other industrial chemicals. The boiling point is a key factor in the design and optimization of these chemical processes.
-
Laboratory Applications: In laboratory settings, the boiling point of benzoic acid is used as a reference point for calibrating thermometers and other temperature-measuring devices.
Numerical Examples and Data Points
- Boiling Point at Different Pressures:
- At 1 atm (760 mmHg): 250 °C (482 °F; 523 K)
- At 0.5 atm (380 mmHg): 239 °C (462 °F; 512 K)
-
At 2 atm (1520 mmHg): 263 °C (505 °F; 536 K)
-
Vapor Pressure of Benzoic Acid:
- At 20 °C: 0.0012 mmHg
- At 100 °C: 7.5 mmHg
-
At 200 °C: 380 mmHg
-
Enthalpy of Vaporization:
-
ΔHvap = 51.2 kJ/mol
-
Boiling Point Elevation:
-
In a 0.1 M solution of benzoic acid in water, the boiling point elevation is approximately 0.052 °C.
-
Melting Point and Boiling Point Comparison:
- Melting point of benzoic acid: 122 °C (252 °F; 395 K)
- Boiling point of benzoic acid: 250 °C (482 °F; 523 K)
These data points and numerical examples provide a comprehensive understanding of the boiling point of benzoic acid and its relationship with other physical properties.
Conclusion
The boiling point of benzoic acid, 250 °C (482 °F; 523 K) at standard atmospheric pressure, is a well-defined and crucial physical property of this organic compound. Understanding the factors that influence the boiling point, such as molecular structure, pressure, and impurities, is essential for the effective synthesis, purification, and application of benzoic acid in various industries. The practical implications of the boiling point, including its role in recrystallization, distillation, and sublimation processes, as well as its importance in food preservation, pharmaceutical manufacturing, and chemical synthesis, make it a valuable parameter for scientists and engineers working with this versatile compound.
Reference:
- Benzoic Acid Boiling Point
- Benzoic Acid on Wikipedia
- Benzoic Acid on PubChem
- Clausius-Clapeyron Equation
- Enthalpy of Vaporization of Benzoic Acid
- Boiling Point Elevation

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.