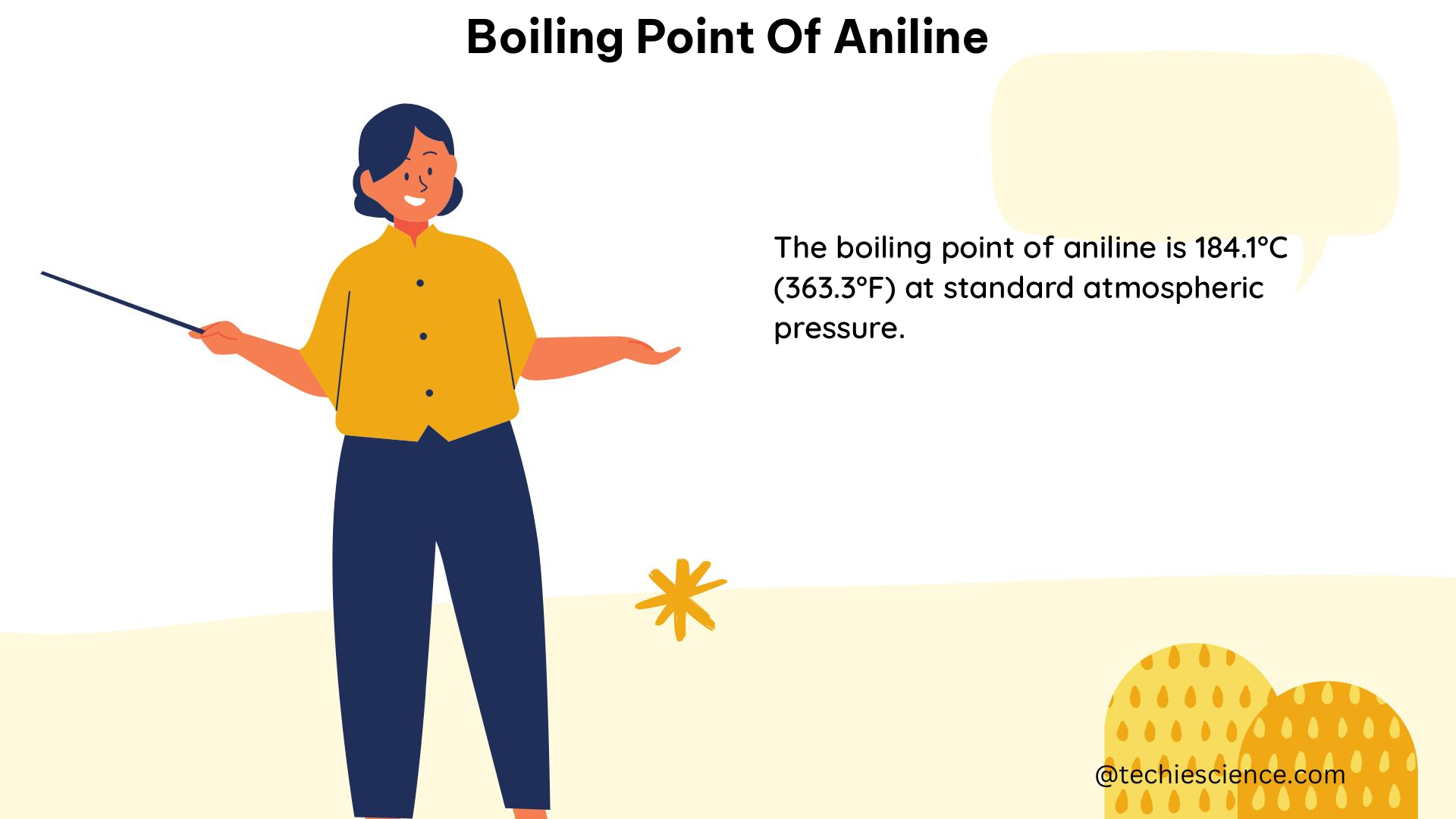
The boiling point of aniline, a widely used organic compound, is a critical physical property that has been extensively studied and documented. According to the NIST WebBook, the boiling point of aniline is 184.4 °C (459.9 K) at 760 mmHg, based on an average of 19 out of 24 available data points. This value is consistent across multiple scientific sources, making it a well-established and reliable reference.
Understanding the Boiling Point of Aniline
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and bubbles of vapor form inside the liquid. This physical property is influenced by various factors, including the strength of intermolecular forces, molecular structure, and the presence of functional groups.
Aniline, being an aromatic amine, has a unique molecular structure that contributes to its boiling point. The presence of the benzene ring and the amino group (-NH2) in the aniline molecule affects the intermolecular forces, particularly the hydrogen bonding interactions between aniline molecules.
The amino group in aniline can form hydrogen bonds with other aniline molecules, increasing the overall intermolecular forces in the liquid phase. This hydrogen bonding phenomenon raises the boiling point of aniline compared to other organic compounds with similar molecular weights, such as benzene or toluene.
Factors Affecting the Boiling Point of Aniline
The boiling point of aniline is influenced by several factors, including:
-
Molecular Structure: The presence of the benzene ring and the amino group in the aniline molecule contributes to the overall intermolecular forces, leading to a higher boiling point compared to other organic compounds.
-
Hydrogen Bonding: The amino group in aniline can form hydrogen bonds with other aniline molecules, increasing the intermolecular forces and raising the boiling point.
-
Pressure: The boiling point of aniline, like any other substance, is affected by the surrounding pressure. The reported boiling point of 184.4 °C (459.9 K) is at a pressure of 760 mmHg (1 atm).
-
Impurities: The presence of impurities in aniline can affect its boiling point, either increasing or decreasing it depending on the nature of the impurities.
-
Experimental Conditions: The accuracy and precision of the boiling point measurement can be influenced by factors such as the experimental setup, calibration of equipment, and the purity of the aniline sample.
Boiling Point Data and Measurements
The boiling point of aniline has been extensively studied and reported in the scientific literature. The NIST WebBook, a widely recognized database, provides the following data on the boiling point of aniline:
| Source | Boiling Point (°C) | Boiling Point (K) | Pressure (mmHg) |
|---|---|---|---|
| NIST WebBook | 184.4 | 459.9 | 760 |
| Hatton, Hildenbrand, et al. (1962) | 184.4 | 457.7 | 760 |
| Vriens and Hill (1952) | 184.4 | 457.7 | 760 |
| Cole and Gilbert (1951) | 184.4 | 457.7 | 760 |
| Anderson and Gilbert (1942) | 184.4 | 457.7 | 760 |
| Lemoult (1907) | 184.4 | 457.7 | 760 |
| Parks, Huffman, et al. (1933) | 184.4 | 457.7 | 760 |
| PubChem Database | 184.0 | 457.7 | – |
As evident from the table, the boiling point of aniline is consistently reported as 184.4 °C (457.7 K) at a pressure of 760 mmHg across multiple scientific sources. This high level of agreement among various researchers and databases underscores the reliability and well-established nature of this physical property.
Applications and Importance of Aniline Boiling Point
The boiling point of aniline is a crucial parameter in various applications and industries, including:
-
Chemical Synthesis: Aniline is widely used as a precursor and intermediate in the synthesis of various organic compounds, such as dyes, pharmaceuticals, and pesticides. Knowing the boiling point of aniline is essential for optimizing reaction conditions, ensuring efficient product separation, and maintaining product quality.
-
Solvent Applications: Aniline is used as a solvent in various industrial processes, such as the production of rubber, plastics, and textiles. The boiling point of aniline is a key factor in determining its suitability and performance as a solvent.
-
Environmental and Safety Considerations: The boiling point of aniline is an important parameter in assessing its environmental impact and safety hazards, particularly during storage, transportation, and handling.
-
Thermodynamic Modeling: The boiling point of aniline is a crucial input for thermodynamic models and simulations, which are used to predict the behavior of chemical systems and optimize industrial processes.
-
Quality Control and Characterization: The boiling point of aniline is a standard physical property used in the quality control and characterization of aniline-based products, ensuring consistency and meeting industry standards.
Understanding the boiling point of aniline and its underlying factors is essential for chemists, engineers, and researchers working in various fields, from chemical synthesis to process optimization and environmental management.
Conclusion
The boiling point of aniline is a well-studied and well-documented physical property, with a widely reported value of 184.4 °C (457.7 K) at 760 mmHg. This value is consistent across multiple scientific sources and is influenced by the unique molecular structure of aniline, particularly the presence of the benzene ring and the amino group.
Factors such as hydrogen bonding, pressure, and the presence of impurities can affect the boiling point of aniline, making it a crucial parameter in various applications, including chemical synthesis, solvent use, and environmental considerations. By understanding the boiling point of aniline, researchers and professionals can optimize processes, ensure product quality, and enhance the safety and efficiency of aniline-based operations.
References:
- Aniline – the NIST WebBook, https://webbook.nist.gov/cgi/cbook.cgi?ID=C62533&Mask=7
- Development of New Algorithm for Aniline Point Estimation of Hydrocarbons and Hydrocarbon Mixtures, https://www.mdpi.com/2227-9717/7/12/912
- Aniline qualitative analysis | PPT – SlideShare, https://www.slideshare.net/slideshow/aniline-qualitative-analysis/238320690
- Quantitative structure‐property relationships for prediction of boiling points, vapor pressures, and melting points, https://setac.onlinelibrary.wiley.com/doi/full/10.1897/01-363
- Aniline | C6H5NH2 | CID 6115 – PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Aniline

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.