Beryllium chloride is a colourless solid that is hygroscopic and easily dissolves in many polar solvents. Let us go into detail about its facts.
Beryllium chloride is a chemical compound with the formula BeCl2. Its properties are similar to those of aluminium chloride because of the diagonal interaction between beryllium and aluminium. Chloride compounds can be converted into chlorine gas and metal through the electrolysis of BeCl2.
Let us talk about the characteristics of Beryllium chloride(BeCl2), such as its IUPAC name, molar mass, density, covalent radius, and whether it is basic or acidic.
Beryllium chloride IUPAC name
BeCl2 is also known as beryllium dichloride, according to the IUPAC name.
Beryllium chloride chemical formula
The chemical formula of beryllium chloride is BeCl2.
Beryllium chloride CAS number
The CAS registration number for beryllium chloride(BeCl2) is 7787-47-5.
Beryllium chloride ChemSpider ID
The ChemSpider ID for beryllium chloride(BeCl2) is 22991, which is specific to each molecule.
Beryllium chloride chemical classification
The following categories are used to classify beryllium chloride(BeCl2):
- Beryllium chloride(BeCl2) is an inorganic chloride compound.
- Beryllium chloride(BeCl2) is classified as a Strategic and vital material.
- Beryllium chloride(BeCl2) is sp3 hybridised in solid form and sp hybridised in gaseous form.
- Beryllium chloride(BeCl2) establishes a single covalent bond with a linear structure.
- The beryllium chloride(BeCl2) molecule’s bond angle is 180°.
Beryllium chloride molar mass
Beryllium chloride(BeCl2) has a molar mass of 79.9182 a.m.u (atomic mass units).
Beryllium chloride color
Beryllium chloride(BeCl2) is a white to slightly yellow powder or colourless molecule.
Beryllium chloride viscosity
The viscosity of Beryllium chloride(BeCl2) is low because it is a crystalline solid substance having no colour.
Beryllium chloride molar density
The molar density of solid beryllium chloride(BeCl2) is 1.899 g/cm3.
Beryllium chloride melting point
Beryllium chloride(BeCl2) has a melting point of 399 °C (750 °F; 672 K). For such a tiny covalent molecule, the melting point is relatively high.
Beryllium chloride boiling point
Beryllium chloride(BeCl2) has a boiling point of 482 °C (900 °F; 755 K).
Beryllium chloride state at room temperature
BeCl2, or beryllium chloride, is solid at normal temperature.
Beryllium chloride covalent bond
Beryllium chloride(BeCl2) has only a single covalent bond. Because beryllium’s small atom has a high relative ionisation energy (900 kJ/mol), which prohibits it from generating cations, this is primarily the cause. It prefers to attract an electron pair that is in a bond by itself.
Beryllium chloride covalent radius
It is impossible to calculate the covalent radius for beryllium chloride(BeCl2) because it can only be calculated for a single atom. The covalent radius of the chlorine atom is 102 pm, while the radius of the beryllium atom is 112 pm.
Beryllium chloride electron configurations
A material’s configuration of electrons inside its atomic orbitals is called its electronic configuration. Let us study the electronic structure of Beryllium chloride(BeCl2).
- The Beryllium atoms in Beryllium chloride(BeCl2) have the electronic configurations [He]1s22s2 & [He]1s22s12p1in the ground state and excited state, respectively.
- The chlorine atom in Beryllium chloride(BeCl2) has the electronic configuration [Ne]3s23p5.
Beryllium chloride oxidation state
In Beryllium chloride(BeCl2), the total oxidation state is 0. Be has an oxidation value of +2 in BeCl2. Cl has an oxidation value of -1 in BeCl2. As a result, the molecule has a charge-to-mass ratio of 1:2.
Beryllium chloride acidity/alkaline
- Beryllium chloride(BeCl2) has an acidic nature because 4 water molecules coordinate with the Be2+ cation during the dissolution of BeCl2 in water.
- The O-H bond breaks off and releases a proton into the solution as the O-Be bond becomes stronger and stronger.
- The following reaction takes place,
- BeCl2 + 2H2O → Be(OH)2 + 2H+
Is Beryllium chloride odourless?
Beryllium chloride(BeCl2) has a pungent, harsh odour.
Is Beryllium chloride paramagnetic?
Paramagnetism exists in atoms and molecules that have unpaired electrons in their valency shells. Let us discuss Beryllium chloride’s(BeCl2) magnetic behaviour.
Beryllium chloride(BeCl2) does not exhibit magnetic behaviour, due to the utilisation of two of Be’s valence electrons in the creation of bonds with the 2 Cl atoms. In the absence of electrons, it is not possible to explain its magnetic behaviour.
Beryllium chloride hydrates
- The Beryllium chloride(BeCl2) molecule forms a tetrahydrate of chloride such as BeCl2•4H2O([Be(H2O)4]Cl2).
- [Be(H2O)4]2+ and chloride ions are produced along with hydrated beryllium ions.
- Tetraaquaberyllium ions, which are the hydrated beryllium ions, are extremely acidic.
- BeCl2 hydrolyzes in an alkaline medium as follows:
BeCl2 + NaOH → [Be(OH)4]2-
BeCl2 + H2O → [Be(H2O)2]2++ 2Cl–
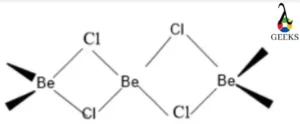
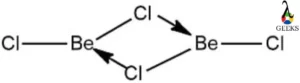
Beryllium chloride crystal structure
- Beryllium chloride(BeCl2) exists in a hexagonal crystal structure. It exists in 2 different polymorphic forms.
- Both structures are based on tetrahedral Be2+ centres and are connected by 2 pairs of chloride ligands.
- BeCl2 has a polymeric chain structure when it is solid. Around the Be atom, 4 Cl atoms form a tetrahedron with 2 covalent bonds and 2 coordinate bonds connecting them.
Beryllium chloride polarity and conductivity
In addition to being non-polar, Beryllium chloride(BeCl2) is conductive because of the following reasons:
- The molecule of beryllium chloride(BeCl2) is non-polar.
- The Be-Cl bond, despite being polar and possessing some net dipole, lacks polarity because the dipole moments of the 2 Be-Cl bonds are equal and opposite, cancelling each other out to generate 0 dipole moment.
- Linearly, the Be-Cl bonds are separated from one another by 180°.
- Beryllium chloride(BeCl2) can conduct electricity when it is in a fuse condition or when it is dissolved in water.
Beryllium chloride reaction with acid
The Beryllium chloride(BeCl2) reaction of acid is extremely minimal because BeCl2 is itself acidic, but reacts with organic protic solvents and water molecules.
BeCl2 + acid → No reaction.
Beryllium chloride reaction with base
- The Beryllium chloride(BeCl2) reacts with bases often as LiAlH4.
- Lewis acid BeCl2 forms beryllium hydride or beryllium hydroxide when it interacts with a base.
BeCl2 + LiAlH4 → BeH2 + LiCl + AlCl3
BeCl2 + NaOH → Be(OH)2 + NaCl
Beryllium chloride reaction with oxide
BeCl2 experiences an acid-base reaction when there is oxide present. The reaction is as follows
BeCl2 + MgO → BeO + MgCl2
Beryllium chloride reaction with metal
Lewis acid BeCl2 undergoes a redox reaction when it interacts with metal. Aqueous Beryllium nitrate and Silver chloride powder are created when dissolved beryllium chloride and dissolved Silver nitrate in water react.
BeCl2 + 2Na → 2NaCl + Be
BeCl2 + Mg → MgCl2 + Be
BeCl2 + AgNO3 → Be(NO3)2 + AgCl
Conclusion
Beryllium chloride(BeCl2) is created when elemental beryllium reacts with chlorines at high temperatures to prepare it. To create beryllium metal through electrolysis, beryllium chloride(BeCl2) and NaCl are utilised as electrolytes.
Read more following properties

Hi….I am Monika. I have done Masters in Chemistry. I am a Subject Matter Expert in Chemistry. I would say that I am a very passionate writer. The main goal of my writing is to present new perspectives. I want to discover new things that I can apply to my surroundings.
Let’s connect through LinkedIn