Benzoic acid is an organic compound. Let us study some facts about benzoic acid.
Benzoic acid is a crystalline white solid, which is the simplest aromatic acid. Benzoic acid is used in a number of industries mainly due to its property of providing preservation for the long term. It is obtained from a variety of natural sources and also acts as an intermediate in reactions that include biosynthesis.
Let us discuss below some more details about Benzoic acids, such as IUPAC name, color, density, and reaction with metals.
Benzoic Acid IUPAC Name
The IUPAC(International Union of Pure and Applied Chemistry) of benzoic acid is Benzoic acid or Benzenecarboxylic acid.
Benzoic Acid Chemical Formula
Benzoic acid has the chemical formula of C6H5COOH while the empirical formula of benzoic acid is C7H6O2. The benzoic acid formula contains seven carbon atoms, 6 hydrogen atoms, and two oxygen atoms.
Benzoic Acid CAS Number
The CAS number(authentic numeric identifier which can contain upto10 digits) of benzoic acid is 65-85-0.
Benzoic Acid Chemspider ID
The ChemSpider ID(ChemSpider is a free chemical structure database) for benzoic acid is 238.
Benzoic Acid Chemical Classification
Benzoic acid is chemically classified as the simplest aromatic carboxylic acid. The benzene ring of the benzoic acid is linked with the carbon of carboxyl group.
Benzoic Acid Molar Mass
The molar mass(mass of one mole of a substance) of benzoic acid is 122.123 g/mol.
Benzoic Acid Color
Benzoic acid is colorless or white solid.
Benzoic Acid Viscosity
The viscosity of benzoic acid is 1.26 mPa (130oC).
Benzoic Acid Molar Density
The molar density of benzoic acid is 0.0088 mol/cm3, and the density of benzoic acid is 1.0749 g/cm3.
Benzoic Acid Melting Point
The melting point of benzoic acid is 122 °C (395 K) or 252 o F.
Benzoic Acid Boiling Point
The boiling point of benzoic acid is 250 °C (523 K) or 482 °F.
Benzoic Acid State at Room Temperature
At room temperature, benzoic acid appears as a fine thread-like crystalline solid. It is insoluble in water and slightly soluble in cold water but is easily soluble in organic solvents.
Benzoic Acid Covalent Bond
The benzene ring in the benzoic acid molecule consists of alternate single and double covalent bonds between the carbon atoms of the rings, the carbon of the carboxyl group forms a covalent bond with one of the carbons of the benzene ring, one with the oxygen and one with the hydroxyl group oxygen atom.

Benzoic Acid Covalent Radius
The covalent radius of benzoic acid cannot be determined as the covalent radius can only be calculated for any single atom.
Benzoic Acid Electron Configurations
Electronic configurations show how the distribution of electrons in the orbitals of an atom takes place. Let us discuss the electronic configuration of benzoic acid in detail.
The electronic configuration of carbon is written as [He] 2s2 2p2. For oxygen, the electronic configuration is written as [He] 2s2 2p4. The electronic configuration of Hydrogen is 1s1.
Benzoic Acid Oxidation State
The oxidation state of all the carbons in benzoic acid is +3. The oxygen of the carboxyl group has a -2 charge, and the hydroxyl hydrogen has +1 an oxidation state.
Benzoic Acid Acidity/Alkaline
Benzoic acid is a weak organic acid with a pKa value of 4.19. The sodium salt of benzoic acid, known as sodium benzoate is highly soluble in water.
Is Benzoic Acid Odourless?
Benzoic acid has a pleasant faint odor.
Benzoic Acid Crystal structure
Benzoic acid has a monoclinic crystal structure and planar molecular shape. The space group of Benzoic acid is P21/n with lattice parameters as a = 7.101Ao, b = 25.48Ao, and c= 7.730Ao.
Benzoic Acid Polarity and Conductivity
Benzoic acid consists of a polar carboxylic group that contains two electronegative oxygen atoms, but the bulk amount of Benzoic acid is nonpolar. It is insoluble in water but is soluble in organic solvents. Benzoic acid is not a good conductor due to the low degree of dissociation in the molten state.
Benzoic Acid Reaction With Acid
Benzoic acid reacts with acids and produces a number of products and water. It reacts with fuming sulfuric acid and undergoes a sulfonation reaction. In this reaction, the hydrogen atom present on the ring carbon, which is meta to the carboxylic group, is replaced by the SO3H functional group.
C6H5COOH + H2SO4 = m-SO3H-C6H5COOH

Benzoic Acid Reaction With Base
Benzoic acid consists of a carboxylic group that reacts with a base and produces a salt. When benzoic acid reacts with sodium hydroxide, sodium benzoate is formed. When sodium benzoate reacts with an acid, it gives the reactant back again. Benzoic acid is toxic, but sodium benzoate is very less toxic.
C6H5COOH + NaOH =C6H5COO–Na+

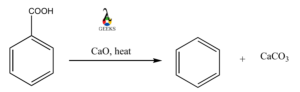
Benzoic Acid Reaction With Oxide
When benzoic acid reacts with oxides, a reduction reaction takes place, which produces a benzene ring. For example, when benzoic acid is reacted with calcium oxide, the acid is reduced to a benzene ring and produces calcium carbonate as the side product of the reaction.
C6H5COOH + CaO + Heat = C6H6 + CaCO3
Conclusion
Benzoic acid was formerly known as gum benzoin. It is mainly used in the production of phenol and in curing many skin-related ailments. Benzoic acid mainly gives an electrophilic substitution reaction as it contains an electron-withdrawing carboxylic group. Benzoic acid is meta-directing.

Hello everyone, I am Aparna Kushwaha. I have completed my Master’s in Chemistry from the University of Lucknow and doing a Ph.D. in Nanoparticles from the same institute. I have worked as an SME for an ed-tech company for 8 months. Currently, I am working as a Subject Matter Expert with Lambdageeks.