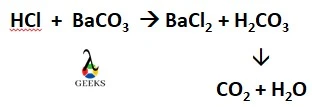15 Facts on HCl + BaCO3: What, How To Balance & FAQs
BaCO3 is an alkali metal carbonate and HCl is an inorganic acid. Let us explore how these two compounds are reacting to each other. HCl, a strong mineral acid, reacts with BaCO3, a basic salt leading to the formation of a salt and acid first. The product acid is immediately decomposed into a gas and … Read more