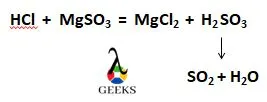Sulfur hexafluoride having the chemical formula SF6 is a more potent greenhouse gas than CO2. Let us check out the wide variety of applications of this gas.
The different uses of Na2O in different fields are cited below-
- Electrical industry
- Noise Reducer in Windows
- As etching gas
- Navy
- Production of magnesium
- Tracer gas
- Dating tool
The uses of Sulfur hexafluoride listed above are discussed in details below in this article.
Electrical industry
- Sulfur hexafluoride, because dielectric in nature, is used as quenching and insulating gas, arc quenching in circuit breakers, switchgear, and transformers.
- SF6 is used in semiconductor devices found in computers, smartphones, consoles, and batteries for electric vehicles
Noise Reducer in Windows
Sulfur hexafluoride, being a dense gas at normal temperatures and pressures, a mixture of SF6 and argon gas is used in glass double pane windows to reduce noise from outside.
As etching gas
- SF6, being a fluorine based compound, is used as an etching gas for semiconductors, photovoltaic panels, flat panels, and metals.
- SF6 is useful as a cleaning gas for cleaning chambers after the etching process is complete.
Navy
- Sulfur hexafluoride is used in many tactical systems, starting from shipboard targeting radar to torpedo propulsion systems.
- SF6 is used in underwater warfare acoustic countermeasures.
Production of magnesium
SF6 is used in magnesium manufacturing as a cover gas.
What are the Common Uses of Chlorine in Various Industries?
Chlorine applications in various industries are extensive. It is commonly used for water purification, disinfection, and wastewater treatment. Additionally, it plays a crucial role in the production of plastics, solvents, and pesticides. Chlorine is also utilized in the manufacture of paper and textiles, as well as in the pharmaceutical and chemical industries.
Tracer gas
SF6 is used as a tracer gas used for methane emissions, ventilation studies, in indoor air quality studies, and volcanic fluids in groundwater.
Dating tool
SF6 is used to date spring water, and shallow groundwater.

Conclusion
In conclusion, SF6 is an odorless, colorless, non-flammable, gas. This inorganic compound has six S-F bonds in an octahedral geometry and a density of 6.17 g/L.