15 Facts on H2SO4 + Sr(NO3)2: What, How To Balance & FAQs
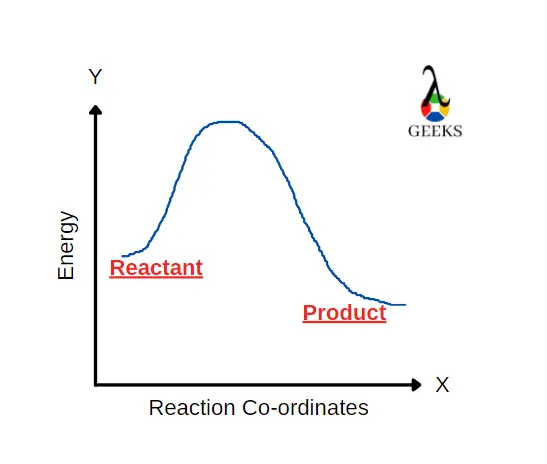
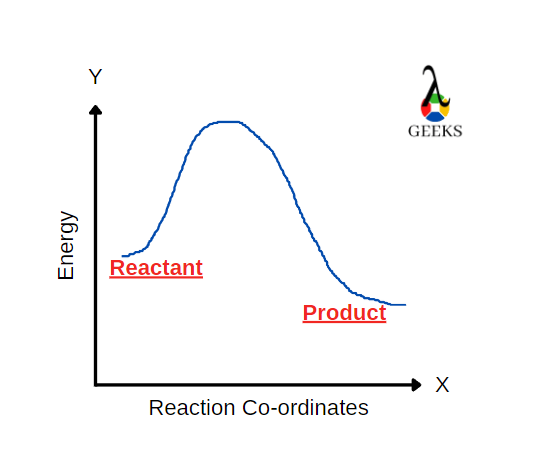
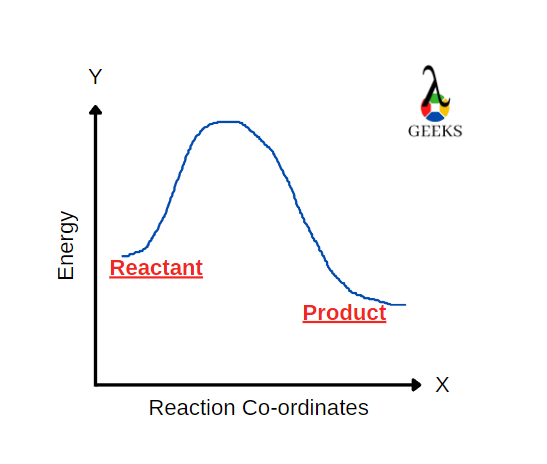
Strontium nitrate [Sr(NO3)2] is an inorganic compound composed of nitrogen, oxygen and strontium. Let us look at how Sr(NO3)2 reacts with H2SO4 in this article. H2SO4 reacts with Sr(NO3)2 to form a salt and an acid. H2SO4 is a strong acid and a commonly used dehydrating agent in organic chemistry. Only P2O5 is the strongest … Read more