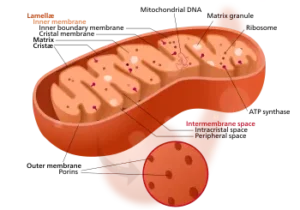
Endonucleases, a class of restriction enzymes, are essential tools in the field of molecular biology, renowned for their ability to recognize and cleave specific DNA sequences. These remarkable enzymes have revolutionized various applications, from genetic engineering to forensic analysis, earning their discoverers the prestigious Nobel Prize in Medicine and Physiology in 1978.
Understanding Endonucleases: The Molecular Workhorses
Endonucleases are enzymes that can recognize and cut DNA molecules at specific nucleotide sequences, known as recognition sites. These recognition sites are typically 4 to 8 base pairs long and are often palindromic, meaning they read the same forward and backward. When an endonuclease binds to its recognition site, it creates a double-strand break in the DNA, either leaving blunt ends or generating staggered cuts that result in single-stranded overhangs.
The discovery of these remarkable enzymes is credited to the pioneering work of three scientists: Werner Arber, Hamilton O. Smith, and Daniel Nathans. Their groundbreaking research on the mechanisms of bacterial defense systems against viral infections led to the identification and characterization of these DNA-cleaving enzymes, earning them the 1978 Nobel Prize.
Types of Endonucleases: Diversity and Specificity

Endonucleases can be classified into several types based on their recognition sequences, cleavage patterns, and cofactor requirements. Some of the most common types include:
-
Type I Endonucleases: These enzymes recognize and cleave DNA at sites distant from their recognition sequence, requiring ATP and a cofactor, such as S-adenosylmethionine (SAM), for their activity.
-
Type II Endonucleases: This is the most widely used class of endonucleases in molecular biology. They recognize and cleave DNA within or adjacent to their specific recognition sequences, typically 4 to 8 base pairs long. Examples include EcoRI, BamHI, and HindIII.
-
Type III Endonucleases: These enzymes recognize and cleave DNA at a fixed distance from their asymmetric recognition sites, requiring ATP and a cofactor for their activity.
-
Type IV Endonucleases: This class of enzymes recognizes and cleaves modified DNA, such as methylated or hydroxymethylated DNA, as a defense mechanism against foreign genetic material.
The high specificity of endonucleases is a crucial feature that makes them invaluable in various applications. Each enzyme recognizes and cleaves a unique DNA sequence, allowing for precise manipulation and analysis of genetic material.
Applications of Endonucleases: Revolutionizing Molecular Biology
Endonucleases have become indispensable tools in the field of molecular biology, enabling a wide range of applications:
-
Genetic Engineering: Endonucleases are essential for DNA cloning, gene insertion, and genetic modification. They allow for the precise excision and insertion of DNA fragments, facilitating the creation of recombinant DNA molecules.
-
DNA Mapping and Sequencing: Endonucleases are used to generate DNA fragments of specific sizes, which can then be analyzed to create detailed genetic maps and facilitate DNA sequencing.
-
Diagnostic and Forensic Analysis: Endonucleases are employed in DNA fingerprinting and forensic investigations, where they are used to generate unique DNA profiles for identification and comparison purposes.
-
Gene Expression Studies: Endonucleases are utilized in techniques like Northern blotting and RNase protection assays to analyze gene expression patterns and quantify specific RNA transcripts.
-
Epigenetic Studies: Certain endonucleases, such as those that recognize and cleave methylated DNA, are employed in the study of epigenetic modifications and their impact on gene regulation.
-
Genome Editing: The development of CRISPR-Cas9 technology, which utilizes a guide RNA and the Cas9 endonuclease, has revolutionized genome editing, allowing for precise and targeted modifications of genetic sequences.
-
Molecular Diagnostics: Endonucleases are used in various molecular diagnostic techniques, such as the Direct Restriction Assay (DRA), which enables the detection and quantification of specific DNA targets with high sensitivity and specificity.
The versatility and precision of endonucleases have made them indispensable tools in the fields of genetics, genomics, and biotechnology, driving advancements in our understanding of the genetic code and enabling groundbreaking applications in medicine, agriculture, and beyond.
Endonucleases in Action: Techniques and Protocols
Researchers and scientists have developed a wide range of techniques and protocols to harness the power of endonucleases effectively. Some of the commonly used methods include:
-
Restriction Fragment Length Polymorphism (RFLP): This technique utilizes endonucleases to generate DNA fragments of varying lengths, which can be separated and analyzed to identify genetic variations or polymorphisms.
-
Southern Blotting: Endonucleases are used to cleave DNA samples, and the resulting fragments are separated by gel electrophoresis, transferred to a membrane, and probed with labeled DNA sequences to detect specific genetic targets.
-
DNA Fingerprinting: Endonucleases are employed in DNA profiling techniques, such as Amplified Fragment Length Polymorphism (AFLP) and Variable Number Tandem Repeat (VNTR) analysis, to generate unique DNA patterns for identification and forensic applications.
-
Methylation-Sensitive Restriction Enzyme (MSRE) Analysis: Certain endonucleases, such as HpaII and MspI, are used to detect and analyze DNA methylation patterns, which play a crucial role in epigenetic regulation.
-
Direct Restriction Assay (DRA): This technique utilizes endonucleases to cleave target-probe hybrids, releasing a molecular marker that can be detected and quantified, enabling the sensitive and specific detection of target DNA sequences.
-
Genome Editing with CRISPR-Cas9: The Cas9 endonuclease, guided by a specific RNA sequence, is used to precisely target and cleave desired DNA sequences, allowing for efficient genome editing and modification.
These are just a few examples of the numerous techniques and protocols that leverage the unique properties of endonucleases to advance scientific research and applications.
Endonucleases: Pushing the Boundaries of Molecular Biology
As the field of molecular biology continues to evolve, the role of endonucleases is expected to expand even further. Ongoing research and technological advancements are unlocking new frontiers in areas such as:
-
Improved Enzyme Engineering: Scientists are exploring ways to engineer endonucleases with enhanced specificity, activity, and stability, expanding their utility in various applications.
-
Multiplexed Genome Editing: The development of CRISPR-Cas9 systems with multiple guide RNAs allows for the simultaneous targeting and modification of multiple genetic loci, revolutionizing genome engineering.
-
Epigenetic Regulation: Endonucleases that recognize and cleave specific epigenetic modifications, such as DNA methylation or histone modifications, are enabling deeper insights into the complex mechanisms of gene regulation.
-
Diagnostic and Therapeutic Applications: The high specificity and sensitivity of endonucleases are being leveraged in the development of advanced molecular diagnostics and targeted therapeutic approaches, such as gene therapy.
-
Synthetic Biology: Endonucleases are playing a crucial role in the design and construction of synthetic genetic circuits, paving the way for the creation of novel biological systems and applications.
As the scientific community continues to push the boundaries of molecular biology, the versatility and precision of endonucleases will undoubtedly remain at the forefront of groundbreaking discoveries and technological advancements.
Conclusion
Endonucleases, the remarkable restriction enzymes, have revolutionized the field of molecular biology, enabling unprecedented control and manipulation of genetic material. From their discovery to their widespread applications, these molecular workhorses have become indispensable tools in the hands of researchers and scientists worldwide.
As the understanding of these enzymes continues to deepen, and new applications emerge, the future of endonucleases in advancing scientific knowledge and addressing global challenges remains bright and full of promise.
References
- Promega Corporation. (n.d.). Restriction Enzyme Resource. Retrieved from https://www.promega.com/resources/guides/nucleic-acid-analysis/restriction-enzyme-resource/
- Guo, J., Ju, J., & Turro, N. J. (2012). Fluorescent hybridization probes for nucleic acid detection. Analytical and Bioanalytical Chemistry, 402(10), 3115-3125. https://doi.org/10.1007/s00216-011-5528-z
- Quizlet. (n.d.). Forensic Analysis: Restriction Enzymes. Retrieved from https://quizlet.com/93432607/forensic-analysisrestriction-enzymes-flash-cards/
- Nature Education. (n.d.). Restriction Enzymes. Retrieved from https://www.nature.com/scitable/topicpage/restriction-enzymes-545/
- ScienceDirect. (n.d.). Restriction Enzyme. Retrieved from https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/restriction-enzyme
- Xu, S. Y., Zhu, Z., Zhang, P., Chan, S. H., Samuelson, J. C., Xiao, J., … & Guan, S. (2007). Discovery of natural nicking endonucleases Nb. BbvCI and Nb. BtsI and engineering of top-strand nicking variants from BbvCI and BtsI. Nucleic Acids Research, 35(14), 4608-4618. https://doi.org/10.1093/nar/gkm496
- Pingoud, A., Fuxreiter, M., Pingoud, V., & Wende, W. (2005). Type II restriction endonucleases: structure and mechanism. Cellular and Molecular Life Sciences CMLS, 62(6), 685-707. https://doi.org/10.1007/s00018-004-4513-1