HCL BAO, also known as HCL Business Analytics and Optimization, is a comprehensive suite of services offered by HCL Technologies to help businesses gain valuable insights from their data. It combines advanced analytics, data management, and business intelligence to enable organizations to make informed decisions and drive growth. With HCL BAO, businesses can leverage data-driven strategies to improve operational efficiency, enhance customer experience, and identify new revenue opportunities.
Key Takeaways
| Service | Description |
|---|---|
| Advanced Analytics | Utilize advanced statistical and predictive modeling techniques to uncover patterns and trends in data. |
| Data Management | Ensure data quality, integrity, and security through effective data governance and management practices. |
| Business Intelligence | Transform raw data into meaningful insights through interactive dashboards, reports, and visualizations. |
| Decision Support | Enable decision-makers with real-time information and analytics to make informed and timely decisions. |
| Revenue Optimization | Identify opportunities to optimize pricing, product mix, and customer segmentation to maximize revenue. |
Please note that the table above provides a concise overview of the key services offered by HCL BAO.
Understanding the Reaction
The reaction between HCl (hydrochloric acid) and BaO (barium oxide) is an interesting chemical reaction that involves the formation of a product. Let’s delve deeper into this reaction and explore its various aspects.
What is the product of HCl and BaO?

When HCl and BaO react, they form a product known as barium chloride (BaCl2). This compound is formed by the combination of one barium ion (Ba2+) and two chloride ions (Cl-) from the hydrochloric acid. The balanced chemical equation for this reaction is:
HCl + BaO → BaCl2 + H2O
What type of reaction is HCl + BaO?
The reaction between HCl and BaO is a double displacement reaction. In this type of reaction, the positive ions from one reactant switch places with the positive ions from the other reactant. In this case, the barium ion (Ba2+) from BaO combines with the chloride ion (Cl-) from HCl to form barium chloride (BaCl2), while the hydrogen ion (H+) from HCl combines with the hydroxide ion (OH-) from BaO to form water (H2O).
How to balance HCl + BaO?
To balance the chemical equation for the reaction between HCl and BaO, we need to ensure that the number of atoms of each element is the same on both sides of the equation. Here’s how we can balance the equation:
2HCl + BaO → BaCl2 + H2O
By adding a coefficient of 2 in front of HCl, we ensure that there are two chloride ions on the product side as well. This balances the equation and satisfies the law of conservation of mass.
HCl + BaO titration
In a titration, a solution of known concentration (the titrant) is added to a solution of unknown concentration (the analyte) until the reaction between the two is complete. In the case of HCl and BaO, titration can be used to determine the concentration of either the acid or the base.
HCl + BaO net ionic equation
The net ionic equation for the reaction between HCl and BaO can be written by removing the spectator ions, which are ions that do not participate in the reaction. In this case, the net ionic equation is:
2H+ + O2- → H2O
This equation represents the essential chemical change that occurs during the reaction, where two hydrogen ions combine with one oxygen ion to form water.
HCl + BaO conjugate pairs
In the reaction between HCl and BaO, there are several conjugate pairs involved. A conjugate pair consists of an acid and its corresponding base, or a base and its corresponding acid. In this reaction, the conjugate pairs are:
- HCl (acid) and Cl- (conjugate base)
- BaO (base) and BaCl2 (conjugate acid)
These conjugate pairs play a crucial role in maintaining the equilibrium of the reaction and ensuring the formation of the desired product.
In conclusion, the reaction between HCl and BaO results in the formation of barium chloride (BaCl2) and water (H2O). This double displacement reaction can be balanced by adjusting the coefficients in the chemical equation. Additionally, the reaction can be studied through titration, and its net ionic equation and conjugate pairs provide further insights into its chemical nature.
Exploring the Properties of the Reaction
HCl and BaO Intermolecular Forces
When examining the properties of the reaction between HCl and BaO, it is important to consider the intermolecular forces at play. HCl, or hydrochloric acid, is a polar molecule due to the electronegativity difference between hydrogen and chlorine. On the other hand, BaO, or barium oxide, is an ionic compound composed of Ba2+ cations and O2- anions. The intermolecular forces between HCl molecules are hydrogen bonding, while the forces between BaO ions are ionic bonding. These differences in intermolecular forces contribute to the unique characteristics of the reaction.
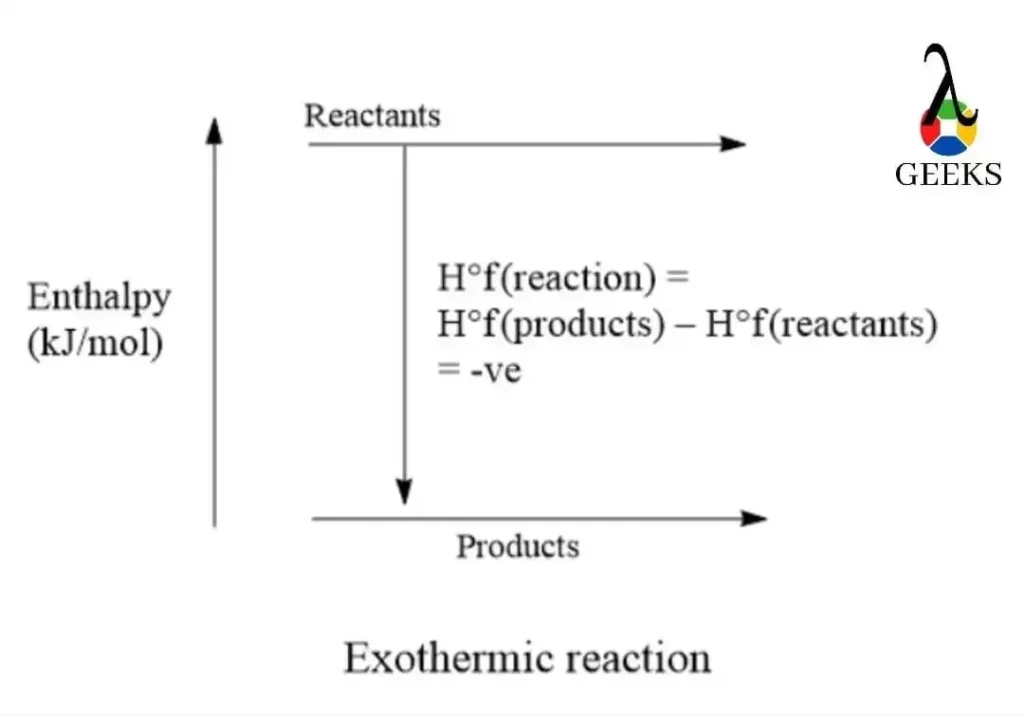
HCl + BaO Reaction Enthalpy
The reaction enthalpy of HCl + BaO is a measure of the heat energy released or absorbed during the reaction. In this case, the reaction is exothermic, meaning it releases heat energy to the surroundings. The enthalpy change is negative, indicating that the reaction is thermodynamically favorable and releases energy. This exothermic nature of the reaction is important to consider when analyzing its properties and potential applications.
Is HCl + BaO a Buffer Solution?
A buffer solution is a solution that resists changes in pH when small amounts of acid or base are added. In the case of HCl + BaO, the reaction does not result in a buffer solution. HCl is a strong acid that completely dissociates in water, while BaO is a basic oxide that reacts with water to form a strong base. The reaction between HCl and BaO does not involve the formation of a weak acid and its conjugate base, which are essential components of a buffer solution. Therefore, HCl + BaO does not exhibit buffer properties.
Is HCl + BaO a Complete Reaction?
A complete reaction refers to a reaction where all the reactants are converted into products, with no excess reactants remaining. In the case of HCl + BaO, the reaction is considered complete when the reactants are in stoichiometric proportions. The balanced chemical equation for the reaction is:
HCl(aq) + BaO(s) → BaCl2(aq) + H2O(l)
The reaction proceeds to completion when the reactants are present in the correct molar ratios. However, if there is an excess of either HCl or BaO, the reaction may not be complete, and some of the reactants may remain unreacted.
Is HCl + BaO an Exothermic or Endothermic Reaction?
As mentioned earlier, the reaction between HCl and BaO is exothermic. This means that the reaction releases heat energy to the surroundings. The negative enthalpy change indicates that the products have a lower energy state than the reactants. The release of heat during the reaction contributes to its exothermic nature. It is important to consider this property when handling and controlling the reaction conditions.
In summary, the reaction between HCl and BaO involves unique intermolecular forces, exhibits exothermic behavior, does not result in a buffer solution, and can be considered complete under specific conditions. Understanding these properties is crucial for further exploration and application of this reaction.
Classifying the Reaction
When studying chemical reactions, it is important to classify them based on their characteristics and properties. This helps us understand the nature of the reaction and predict its behavior. In this section, we will classify the reaction between HCl and BaO and explore various aspects of it.
Is HCl + BaO a redox reaction?
To determine if the reaction between HCl and BaO is a redox reaction, we need to examine the changes in oxidation states of the elements involved. In this case, HCl is a strong acid and BaO is a basic oxide. When they react, the hydrogen in HCl is reduced from an oxidation state of +1 to 0, while the barium in BaO is oxidized from an oxidation state of +2 to +3. Therefore, we can conclude that the reaction between HCl and BaO is indeed a redox reaction.
Is HCl + BaO a precipitation reaction?
A precipitation reaction occurs when two aqueous solutions react to form an insoluble solid, known as a precipitate. In the case of HCl and BaO, both are soluble in water, and no solid precipitate is formed. Therefore, we can conclude that the reaction between HCl and BaO is not a precipitation reaction.
Is HCl + BaO reversible or irreversible reaction?
To determine if the reaction between HCl and BaO is reversible or irreversible, we need to consider the nature of the reaction and the conditions under which it occurs. In this case, the reaction between HCl and BaO is irreversible because it proceeds in one direction only, resulting in the formation of products that cannot readily convert back to the reactants.
Is HCl + BaO a displacement reaction?
A displacement reaction occurs when one element displaces another element from a compound. In the case of HCl and BaO, no displacement occurs as both HCl and BaO are stable compounds. Therefore, we can conclude that the reaction between HCl and BaO is not a displacement reaction.
In summary, the reaction between HCl and BaO is a redox reaction but not a precipitation reaction, irreversible in nature, and does not involve displacement of elements. Understanding the classification of reactions helps us gain insights into their behavior and properties.
Additional Aspects of HCl and BaO
Hydrogen chloride (HCl) and barium oxide (BaO) are two compounds that have interesting properties and play important roles in various chemical reactions. Let’s explore some additional aspects of HCl and BaO.
Does hydrogen chloride have a low boiling point?
Yes, hydrogen chloride has a relatively low boiling point. At standard temperature and pressure, HCl exists as a gas. Its boiling point is -85.05 degrees Celsius (-120.09 degrees Fahrenheit). This low boiling point allows HCl to easily vaporize and form a gas at room temperature.
Is HCl basic or acid?
Hydrogen chloride is an acid. When dissolved in water, it forms hydrochloric acid (HCl(aq)). HCl is a strong acid, meaning it completely dissociates into H+ ions and Cl- ions in aqueous solution. This makes HCl highly reactive and capable of donating protons to other substances.
Why is HCl boiling point so low?
The low boiling point of HCl can be attributed to its molecular structure and intermolecular forces. HCl is a diatomic molecule consisting of one hydrogen atom and one chlorine atom. The relatively weak intermolecular forces between HCl molecules result in a low boiling point. These forces are primarily dipole-dipole interactions, as HCl has a permanent dipole due to the electronegativity difference between hydrogen and chlorine.
Does HCl have a permanent dipole?
Yes, HCl has a permanent dipole. The chlorine atom is more electronegative than the hydrogen atom, causing the shared electron pair to be closer to chlorine. This creates a partial negative charge on the chlorine atom and a partial positive charge on the hydrogen atom, resulting in a permanent dipole moment.
Does HCl or HBr have a higher boiling point?
HCl has a lower boiling point than HBr. This is because the strength of intermolecular forces increases with the size of the atoms involved. Since bromine (Br) is larger than chlorine (Cl), the intermolecular forces in HBr are stronger, leading to a higher boiling point compared to HCl.
When HCl dissolves in water
When HCl dissolves in water, it forms hydrochloric acid (HCl(aq)). The dissolution of HCl in water is an exothermic process, releasing heat. The HCl molecules dissociate into H+ ions and Cl- ions in the aqueous solution. This dissociation allows HCl to exhibit its acidic properties, such as its ability to react with bases and neutralize them.
In summary, HCl and BaO have unique characteristics that make them important in various chemical reactions. HCl is an acid with a low boiling point and a permanent dipole, while BaO is a compound that can undergo displacement reactions. Understanding these additional aspects of HCl and BaO contributes to our knowledge of their behavior and applications in different contexts.
Conclusion
In conclusion, HCL Bao is a fascinating topic that offers a unique perspective on the future of technology. With its innovative approach to computing and its potential to revolutionize various industries, HCL Bao holds great promise. The concept of using biological systems to enhance computational power and efficiency is truly groundbreaking. While there are still many challenges to overcome and further research to be done, the potential benefits of HCL Bao are undeniable. As we continue to explore and develop this technology, we may witness a new era of computing that merges biology and technology in unprecedented ways.
Frequently Asked Questions
1. What is the hoá trị of HCL?
The hoá trị, or valency, of Hydrogen Chloride (HCL) is 1. This is because Hydrogen and Chlorine each have one electron available for bonding, making the total valency 1.
2. Does Hydrogen Chloride have a low boiling point?
Yes, Hydrogen Chloride (HCL) has a relatively low boiling point of -85.05 degrees Celsius. This is due to its simple molecular structure and weak intermolecular forces.
3. What is the reaction between HCL and BaCO3?
The reaction between Hydrogen Chloride (HCL) and Barium Carbonate (BaCO3) produces Barium Chloride (BaCl2), Water (H2O), and Carbon Dioxide (CO2). The balanced chemical equation is: 2HCL + BaCO3 → BaCl2 + H2O + CO2.
4. What is the balanced equation for the reaction between NaOH and HCL?
The balanced equation for the reaction between Sodium Hydroxide (NaOH) and Hydrogen Chloride (HCL) is: NaOH + HCL → NaCl + H2O. This is a neutralization reaction where an acid and a base react to form a salt and water.
5. Where do Bao buns come from?
Bao buns, also known as steamed buns or baozi, originated from China. They are a type of filled bun or bread-like dumpling that is often used in various Chinese cuisines.
6. How to make Bao buns?
Making Bao buns involves creating a dough from flour, yeast, sugar, and water, which is then allowed to rise. The dough is divided into pieces, each of which is filled with a savory or sweet filling, then steamed until fluffy and soft.
7. Is HCL basic?
No, HCL (Hydrogen Chloride) is not basic. It is an acid. When dissolved in water, it forms Hydrochloric Acid, which is a strong acid.
8. What is the role of HCL in medicine?
HCL (Hydrochloric Acid) plays a crucial role in medicine, particularly in the digestive system. It helps break down food in the stomach, kill bacteria, and absorb nutrients. It’s also used in certain medications to adjust the pH.
9. Is HCL a base or an acid?
HCL, or Hydrogen Chloride, is an acid. When it is dissolved in water, it forms Hydrochloric Acid, a strong acid.
10. What are the BAO services offered by HCL Technologies?
HCL Technologies offers Business Aligned IT (BAO) services that aim to align IT processes with business objectives. These services include Business Process Outsourcing, IT Consulting, Business Transformation, IT Infrastructure Services, Digital Transformation, IT Service Management, and Business IT Alignment.