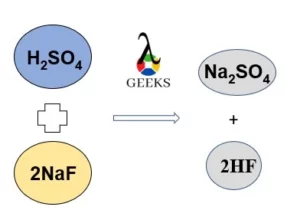
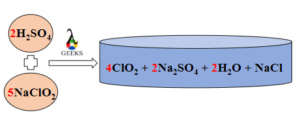
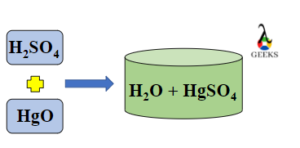
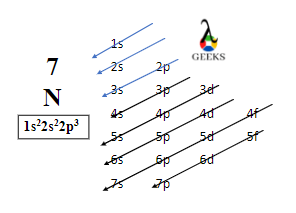
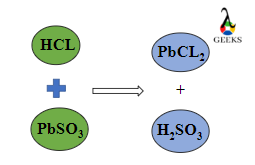
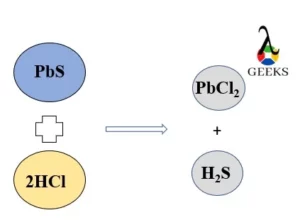
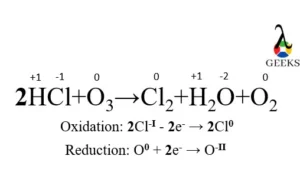
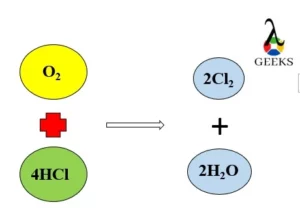
15 Facts on H2SO4+NaF: What, How To Balance & FAQs
Sulphuric acid reacts with natrium fluoride to form the salt cake. Let us study H2SO4 + Naf reaction in detail. Sulfuric acid is a very important industrial chemical. It is usually used in the production of fertilizers and is crucial in chemical synthesis, oil refining, wastewater processing, and mineral processing. Sodium fluoride is a colorless, … Read more