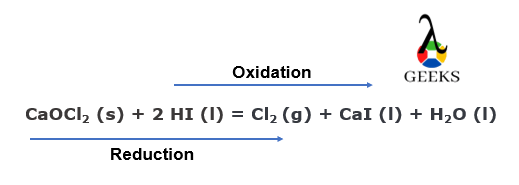
15 Facts on HI + CaOCl2: What, How To Balance & FAQs
Chemical reactions are the interactive process of reactant species in proper orientation to produce new compounds. Let us explore the chemical reactivity of HI and CaOCl2. HI, is a strong acid which is generally a gas under standard conditions. It is used as a reducing agent for various organic syntheses. CaOCl2 is the main component … Read more