The chemical reaction between nitric acid and carbon produces components that are primarily oxidative and responsible for the decomposition of organic substances. Let us explore the chemical reactivity of HNO3 and C.
HNO3 is a colorless corrosive mineral acid that finds various applications in the fertilizer industry. Carbon denoted as C due to its sp2 hybridization can form a variety of interesting compounds.
The reactivity of HNO3 and C can be a source of various important compounds with agricultural benefits. Thus, these important reaction-based incredible facts are presented below in detail:
What is the product of HNO3 and C?
HNO3 and C interact to produce nitrogen dioxide (NO2), Carbon dioxide (CO2), and water (H2O). The complete chemical equation is given as:
HNO3 + C = NO2 + CO2 + H2O
What type of reaction is HNO3 + C?
HNO3 + NaOH is a reduction-oxidation reaction where carbon (C) oxidizes itself to carbon dioxide (CO2), and nitric acid (HNO3) gets reduced to nitrogen dioxide (NO2).
How to balance HNO3 + C?
The following algebraic methodology can be used as an explanation to balance the chemical reaction
HNO3 + C = NO2 + CO2 + H2O,
- Label each species (reactant or product) given in the chemical equation with a respective variable (A, B, C, D, and E) to illustrate unknown coefficients.
- (A) C + (B) HNO3 = (C) CO2 + (D) NO2 + (E) H2O
- Deduce a suitable equation for each element in the reacting species representing the number of atoms of an element in each reactant or product species, applied to solve the equation.
- C = A = C, H = B = 2E, N = B = D, O = 3B = 2C + 2D + E
- The Gaussian elimination and substitution methodology is applied to simplify all the variables and coefficients, and the outcomes are
- A = 1, B = 4, C = 1, D = 4, and D = 2
- Hence, the overall balanced equation is,
- 4 HNO3 + C = 4 NO2 + CO2 + 2 H2O
HNO3 + C titration
HNO3 + C system is not performed as titration as carbon as an element cannot work either as a titre or as a titrant. A specific indicator cannot also be thought of to demonstrate the end point of the titration.
HNO3 + C net ionic equation
The net ionic equation of HNO3 + C is
C (s) + 4 H+ (aq) = 2 H2O (l) + CO2 (g)
The net ionic equation is obtained using the following steps
- Write the balanced chemical equation and designate the physical states of reactants and products accordingly
- HNO3 (aq) + C (s) = 4 NO2 (g) + CO2 (g) + 2 H2O (l)
- Now, strong acids, bases, and salts dissociate into ions whereas pure solid substances and molecules do not dissociate
- H+ + NO3– + C = H+ + NO2– + C+ CO2 + OH–
- Thus, the net ionic equation is
- C (s) + 4 H+ (aq) = 2 H2O (l) + CO2 (g)
HNO3 + C conjugate pairs
- Conjugate pair of strong acid HNO3 is H2NO3+.
- Conjugate pair of C cannot be formed due to unavailability of free hydrogen ions in it.
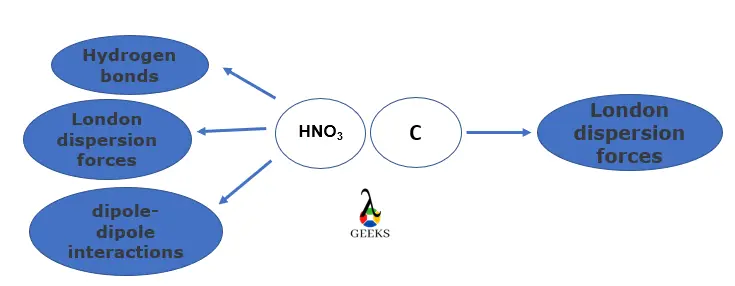
HNO3 and C intermolecular forces
Intermolecular forces acting on HNO3 and C are:
- HNO3 interacts using strong hydrogen bonds, weak London dispersion forces, and dipole-dipole interactions between the molecules.
- C oxidizes to form CO2 where the movement of the electron causes instantaneous dipoles to form London dispersion forces.
- NO2 register formation of dipole dipole and ion-dipole interactions due to negative and positive charges being found in equilibrium in the molecule.
HNO3 + C reaction enthalpy
HNO3 + C exhibits a negative reaction enthalpy of -631.62 kJ/mol. Enthalpy information for the reactants and products involved are as follows:
- Enthalpy of formation for reactant HNO3: -207.36 kJ/mol
- Enthalpy of formation for reactant C: 716.68 kJ/mol
- Enthalpy of formation for product NO2: 33.18 kJ/mol
- Enthalpy of formation for product CO2: -393.50 kJ/mol
- Enthalpy of formation for product H2O: -241.80 kJ/mol
Is HNO3 + C a buffer solution?
HNO3 + C does not form a buffer because the buffer is prepared by the addition of weak acid to the salt of its conjugate base whereas HNO3 is not a weak acid and C is an amphoteric element and not a salt of the conjugate base of HNO3.
Is HNO3 + C a complete reaction?
HNO3 + C is a complete reaction as the stable products namely nitrogen dioxide (NO2), carbon dioxide (CO2), and water (H2O). are produced in the reaction.
Is HNO3 + C an exothermic or endothermic reaction?
HNO3 + C is an exothermic reaction because the calculated reaction enthalpy is observed to be negative. The enthalpy oof formation of reactants is greater than that of products which justifies that heat is released in the process.
Is HNO3 + C a redox reaction?
HNO3 + C is a redox reaction because carbon (C) oxidizes itself to carbon dioxide (CO2), and nitric acid (HNO3) gets reduced to nitrogen dioxide (NO2).
Is HNO3 + C a precipitation reaction?
HNO3 + C is not a precipitation reaction as NO2 and CO2 produced in the reaction are gases that do not settle as a solid precipitate in the reaction.
Is HNO3 + C reversible or irreversible reaction?
HNO3 + C is an irreversible reaction because the formed products in the reaction do not change back into original reactants till the conditions remain similar.
Is HNO3 + C displacement reaction?
HNO3 + C is not a displacement reaction because no new compounds are observed to be formed due to the displacement of a single component or due to double displacement between both the reacting species.
Conclusions
The chemical reaction of HNO3 + C forms nitrogen dioxide and carbon dioxide as the major products that have huge beneficial properties in the production of fertilizers, pesticides, and related industries. The chemicals have been used in the manufacturing of synthetic dyes, fibres and plastics.