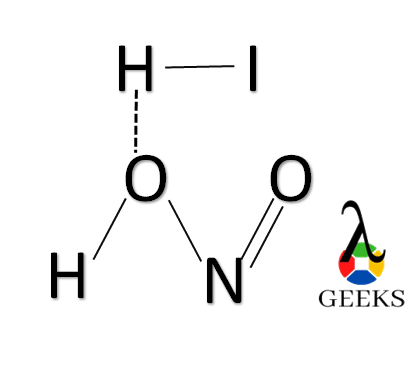
15 Facts on HI + HNO2: What, How To Balance & FAQs
Nitrous acid, also known as HNO2, is a chemical compound that plays a significant role in various industrial and scientific applications. It is an inorganic acid that is commonly used as a reagent in chemical reactions and as a precursor for the synthesis of other compounds. Nitrous acid is a pale blue liquid that is … Read more