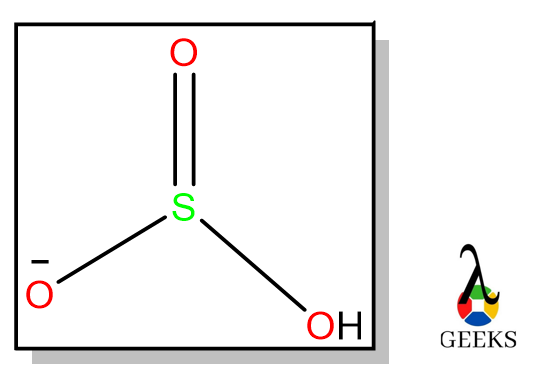
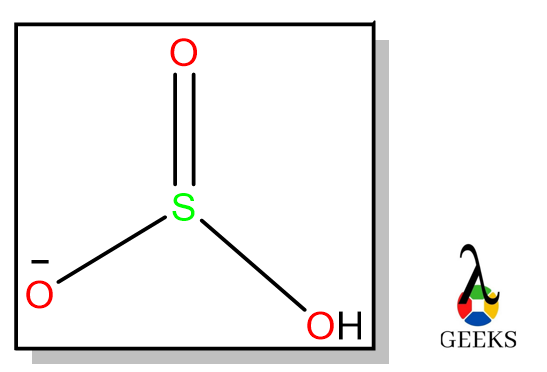
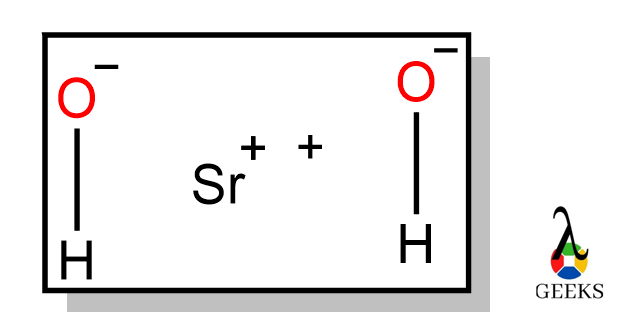
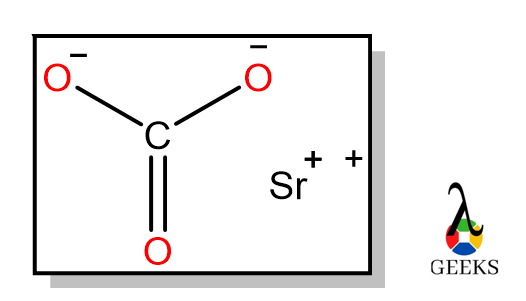
6 hydrochloric Acid Uses: Facts You Should Know!
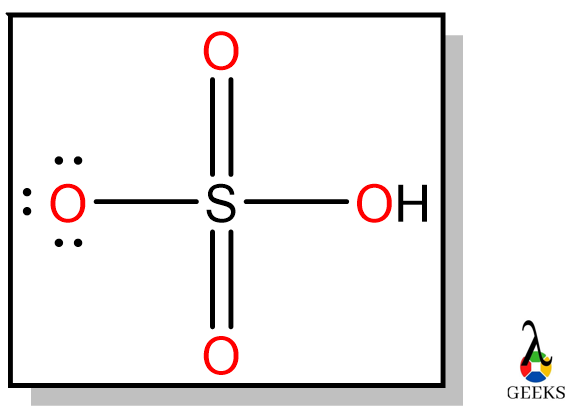
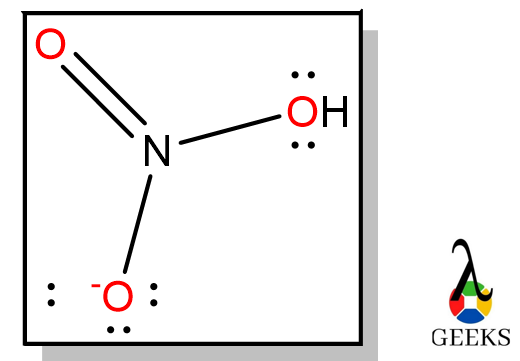
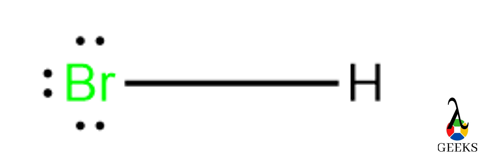
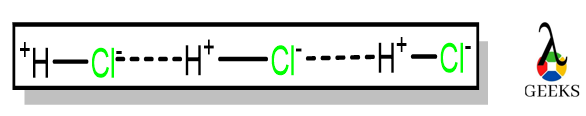
Hydrochloric acid (HCl) is called Chlorohydric acid, and it exists in gaseous form, hydrogen chloride gas. Let us see some facts on HCl acid and its uses. Uses of HCl are Purification of common salt Production of Organic compounds Production of Inorganic compounds Cleansing agents Let us discuss some important facts on Hydrochloric acid and … Read more