Hydrogen Peroxide(H2O2) Properties (25 Facts You Should Know)
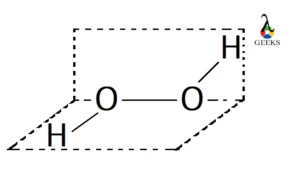
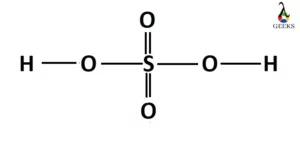
Hydrogen peroxide is an unstable compound that decomposes in presence of light and heat. Let us explore the physical and chemical properties of hydrogen peroxide. Hydrogen peroxide is a reactive oxygen species and becomes miscible in water. It is also soluble in ether. It is stored in a dark bottle (to get rid of light) … Read more