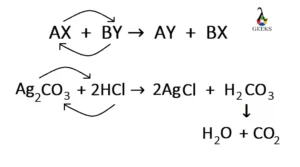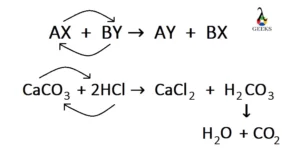15 Facts on H2SO3 + Fe2O3: What, How To Balance & FAQs
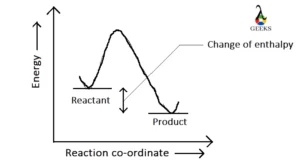
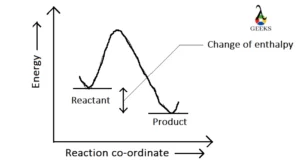
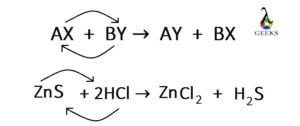
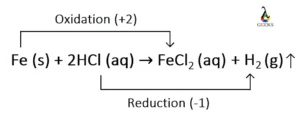
Ferric oxide (Fe2O3) is a transition metal oxide, and it reacts with weak sulfurous acid. Let us explore the products obtained from the reaction of Fe2O3 + H2SO3. Fe2O3 is an amphoteric oxide having both acidic and basic character. Sulfurous acid (H2SO3) is a weak acid, and both of them undergo a neutralization reaction at … Read more